Material selection for advanced engineering relies on a deep understanding of chemical composition and its impact on performance.
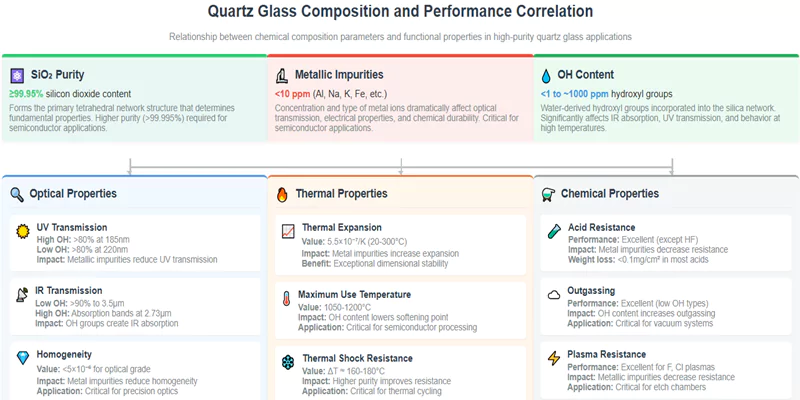
Quartz glass composition consists primarily of ultra-pure silicon dioxide (SiO₂) at >99.95% purity, with trace impurities including metallic elements (Al, Na, K, Fe) and hydroxyl groups (OH) that critically determine optical, thermal, and chemical properties. The specific composition profile—particularly impurity concentrations below 10ppm—directly controls performance in high-precision applications from semiconductor manufacturing to precision optics.
The following sections explore how compositional fundamentals and impurity control underpin the value of quartz glass in demanding environments.
How Does Raw Material Selection Determine Quartz Purity for Precision Applications?
Raw material selection dictates quartz purity through divergent impurity profiles, with synthetic quartz enabling ultra-low contamination essential for semiconductor and photonics applications where natural quartz limitations prove prohibitive.
Raw Material Divergence
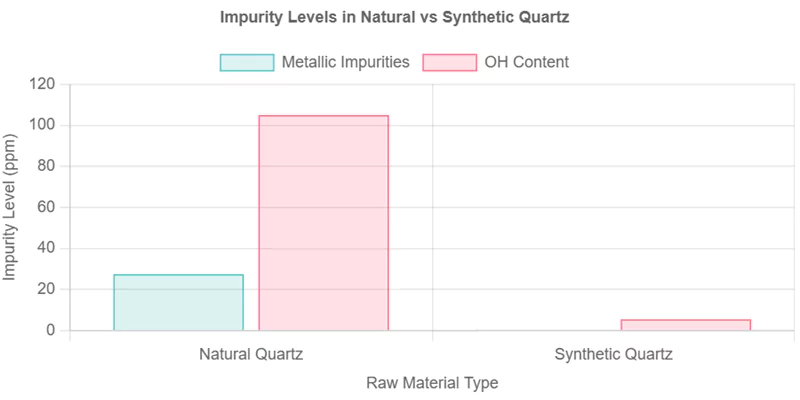
Natural quartz exhibits elevated metallic impurities (e.g., Al, Fe, alkali metals) and hydroxyl (OH) groups due to geological constraints, while synthetic quartz utilizes high-purity precursors (SiCl₄/SiH₄) to achieve ultra-low impurity baselines (<1 ppm metals, controlled OH).
This fundamental dichotomy establishes the impurity profile foundation.
Raw Material Impact on Quartz Glass Composition:
| Raw Material Type | Metallic Impurities (ppm) | OH Content (ppm) | Typical Use Cases |
|---|---|---|---|
| Natural Quartz | 5–50 | 10–200 | General labware, lighting |
| Synthetic Quartz | <1 | <1–10 | Semiconductor, precision optics |
Manufacturing & Purity Control
Synthetic quartz production via flame hydrolysis or chemical Vapor Deposition (CVD) enables precise impurity management.
Process parameters (temperature, precursor purity) suppress metallic contaminants to sub-ppm levels and regulate OH content (±5 ppm), ensuring batch-to-batch consistency unattainable with natural quartz purification.
Manufacturing Method Impact on Composition:
| Method | Metallic Impurities (ppm) | OH Content (ppm) | Notes |
|---|---|---|---|
| Electric Fusion | 1–10 | 10–200 | Higher OH, moderate metals |
| Flame Fusion | <1 | <1–10 | Ultra-low OH, low metals |
| CVD | <0.1 | <1 | Highest purity, costly |
Performance Implications
Ultra-low impurities in synthetic quartz directly enable:
- Optical superiority: >99.8% UV-Vis transmission critical for photolithography masks and EUV optics.
- Thermal resilience: Consistent softening point (~1730°C) for semiconductor crucibles and high-power laser components.
- Defect minimization: Near-zero metallic contaminants prevent devitrification or color center formation in precision optics.
Application-Driven Selection
Ultra-low impurities in synthetic quartz directly enable:
· Synthetic dominance: Semiconductor processing (photomasks, EUV systems), laser optics, and photonics demand impurity-driven performance guarantees.
· Natural applicability: IR-transparent optics tolerate higher OH (e.g., ≤250 ppm) if metallic impurities are controlled; cost-sensitive uses leverage natural quartz where purity thresholds permit.
What is Quartz Glass Composition and Why is Chemical Purity Essential?
Understanding the chemical makeup of quartz glass is fundamental to predicting its behavior in critical applications.
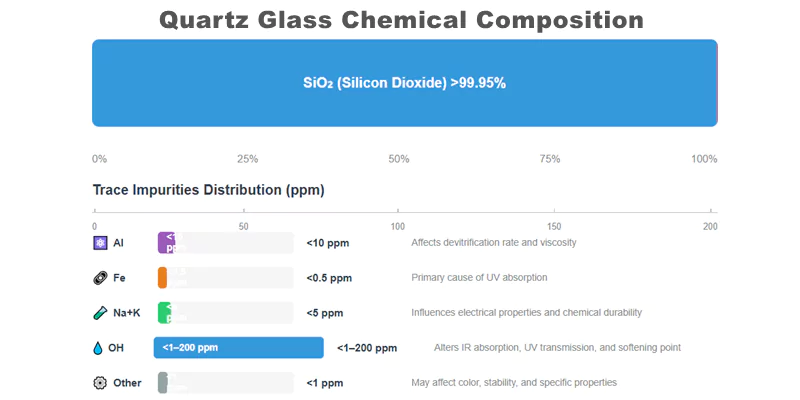
Quartz glass is composed of a continuous silicon dioxide (SiO₂) network, typically exceeding 99.95% purity. Chemical purity is essential because even trace impurities—such as aluminum, iron, sodium, potassium, and hydroxyl groups—can dramatically alter optical transmission, thermal stability, and chemical resistance.
High-purity quartz glass is required for semiconductor, photonics, and laboratory applications where contamination or property drift can cause process failures or measurement errors.
Typical Chemical Composition of Quartz Glass
| Component | Typical Content (ppm) | Role/Impact |
|---|---|---|
| SiO₂ | >999,500 | Network former, determines structure |
| Al | <10 | Affects devitrification, viscosity |
| Fe | <0.5 | Impacts UV absorption |
| Na + K | <5 | Influences electrical properties |
| OH (Hydroxyl) | <1–200 | Alters optical/thermal properties |
| Other Metals | <1 | May affect color, stability |
Why Do Silicon Dioxide Networks Form the Foundation of Superior Properties?
The atomic structure of quartz glass is the basis for its exceptional performance.
A continuous three-dimensional SiO₂ network forms the backbone of quartz glass, resulting in a rigid, amorphous structure with minimal defects. This network imparts high thermal stability, low thermal expansion, and excellent chemical inertness, making quartz glass suitable for extreme environments.
The absence of grain boundaries and the uniformity of the SiO₂ network also contribute to high optical transmission and resistance to devitrification.
Structure-Property Relationship of SiO₂ Networks
| Structural Feature | Resulting Property | Application Benefit |
|---|---|---|
| Continuous Si-O-Si | High thermal stability | Withstands >1000°C |
| Amorphous structure | Low birefringence | Precision optics |
| No grain boundaries | High chemical resistance | Acid/base environments |
| Uniform network | High UV/IR transmission | Spectroscopy, lithography |
What Role Do Trace Impurities Play in Determining Material Characteristics?
Even at parts-per-million levels, impurities can have outsized effects on quartz glass performance.
Trace metallic impurities such as aluminum, iron, sodium, and potassium can disrupt the SiO₂ network, introduce color centers, and catalyze devitrification. Hydroxyl groups (OH) can absorb infrared light and reduce thermal stability. The control of these impurities is thus critical for high-value applications.
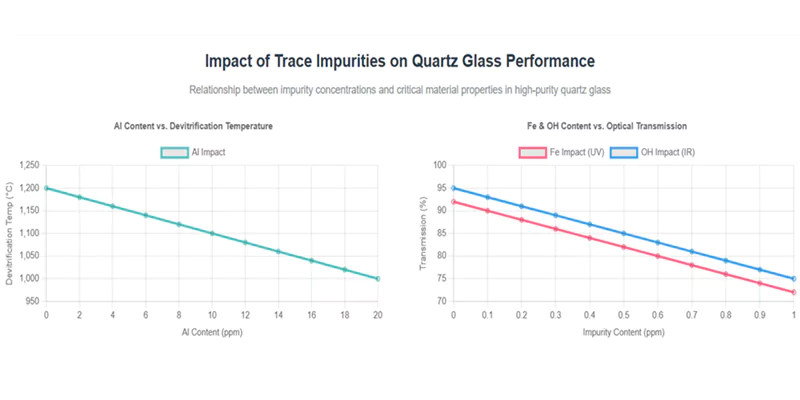
The impact of each impurity depends on its chemical nature, concentration, and the intended application environment.
Effects of Trace Impurities in Quartz Glass
| Impurity | Typical Limit (ppm) | Main Effect | Critical Application Concern |
|---|---|---|---|
| Aluminum (Al) | <10 | Lowers devitrification temp | Furnace tubes, high-temp optics |
| Iron (Fe) | <0.5 | Increases UV absorption | UV optics, photolithography |
| Sodium (Na) | <2 | Reduces electrical resistance | Semiconductor, high-voltage |
| Potassium (K) | <3 | Similar to Na | Same as above |
| OH | <1–200 | Affects IR absorption, stability | IR optics, high-temp processing |
How Do Metallic Impurities Degrade Quartz Glass Stability in High-Temperature Applications?
Maintaining performance at elevated temperatures requires strict control of metallic impurities.
Metallic impurities, especially aluminum and alkali metals, can lower the devitrification temperature of quartz glass, leading to crystallization and loss of transparency or mechanical integrity. Iron and other transition metals can catalyze color center formation and increase absorption losses.
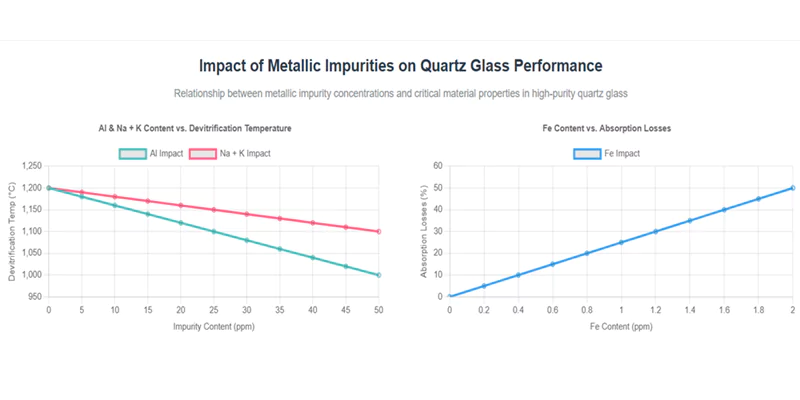
The selection of quartz glass for high-temperature applications must therefore specify not only total SiO₂ content but also individual impurity limits.
Metallic Impurity Impact on High-Temperature Properties
| Impurity | Threshold (ppm) | Effect at 1200°C | Lifetime Reduction (%) |
|---|---|---|---|
| Aluminum (Al) | >20 | Accelerates devitrification | 60–80 |
| Iron (Fe) | >1 | Increases absorption, color | 30–50 |
| Na + K | >5 | Lowers viscosity, increases flow | 20–40 |
Aluminum and Alkali Metal Effects
Aluminum and alkali metals (Na, K) disrupt the SiO₂ network, lowering the viscosity and devitrification temperature. This leads to premature crystallization and mechanical failure in furnace and lamp applications.
Iron and Transition Metal Impacts
Iron and other transition metals introduce absorption bands in the UV and visible spectrum, causing coloration and reduced optical transmission. Even at sub-ppm levels, iron can significantly degrade the performance of UV optics and photolithography components.
How Do Hydroxyl Groups Affect Optical Transmission and Thermal Stability in Quartz Glass?
Hydroxyl (OH) groups are a unique impurity in quartz glass, affecting both optical and thermal properties.
OH groups absorb infrared light, particularly around 2,700–3,600 nm, and can also lower the glass transition temperature. High OH content is detrimental for IR optics and high-temperature applications but may be acceptable for UV applications where IR absorption is less critical.
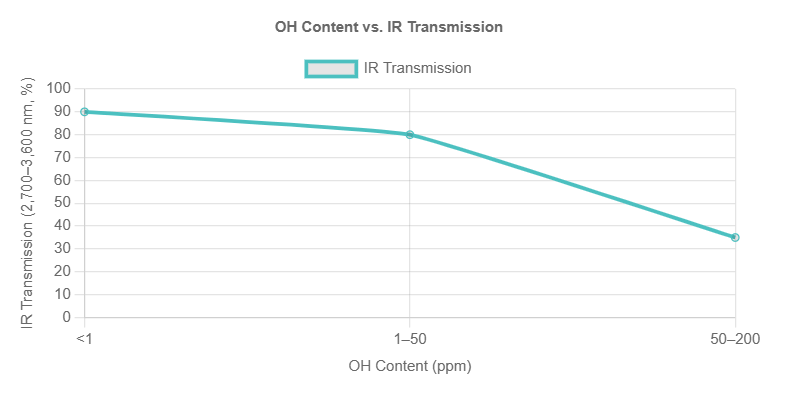
Controlling OH content is achieved through raw material selection and manufacturing process optimization.
Hydroxyl Content and Optical Transmission
| OH Content (ppm) | IR Transmission (2,700–3,600 nm, %) | Suitable Application |
|---|---|---|
| <1 | >90 | IR optics, high-temp furnace |
| 1–50 | 70–90 | General optics, labware |
| 50–200 | <70 | UV optics (if IR not critical) |
What Compositional Features Enable Extreme Environment Applications?
Applications in extreme environments—such as semiconductor fabs, high-power lasers, and chemical reactors—demand quartz glass with tailored compositional features.
Ultra-high purity, low metallic impurity content, and controlled OH levels enable quartz glass to resist devitrification, maintain optical clarity, and withstand aggressive chemicals or high temperatures.
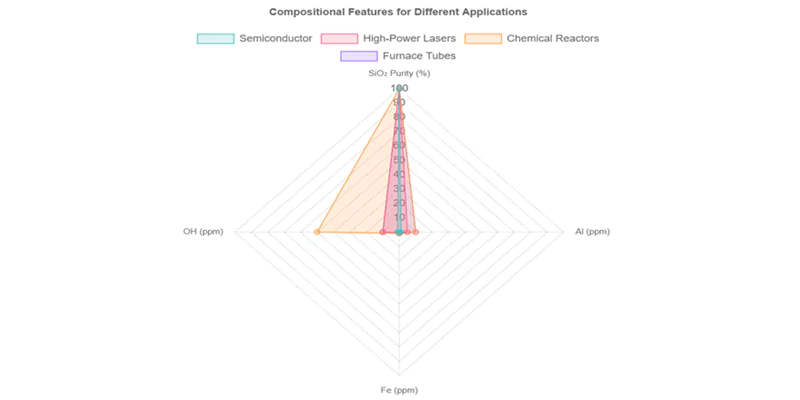
The right compositional profile ensures long service life and consistent performance under demanding conditions.
Compositional Requirements for Extreme Applications
| Application | SiO₂ Purity (%) | Al (ppm) | Fe (ppm) | OH (ppm) | Key Performance Need |
|---|---|---|---|---|---|
| Semiconductor | >99.995 | <1 | <0.1 | <1 | Yield, contamination control |
| High-Power Lasers | >99.99 | <5 | <0.5 | <10 | UV/IR transmission, durability |
| Chemical Reactors | >99.95 | <10 | <1 | <50 | Acid/base resistance |
| Furnace Tubes | >99.95 | <10 | <0.5 | <10 | Thermal shock, devitrification |
Synthetic vs. Natural Quartz Composition: Which Delivers Superior Purity?
The debate between synthetic and natural quartz centers on achievable purity and performance.
Synthetic quartz, produced from high-purity precursors via flame hydrolysis or CVD, consistently delivers lower metallic and OH impurity levels than natural quartz. This makes it the preferred choice for semiconductor, photonics, and other high-precision applications.
Synthetic vs. Natural Quartz: Composition Comparison
| Property | Synthetic Quartz | Natural Quartz |
|---|---|---|
| SiO₂ Purity (%) | >99.995 | 99.90–99.99 |
| Al (ppm) | <1 | 5–50 |
| Fe (ppm) | <0.1 | 0.5–5 |
| OH (ppm) | <1–10 | 10–200 |
| Typical Application | Semiconductor, optics | Lighting, labware |
What Compositional Specifications Determine Optimal Material Selection?
Optimal material selection is guided by application-specific compositional specifications.
Key parameters include SiO₂ purity, individual metallic impurity limits, OH content, and particle inclusion levels. These specifications should be matched to the performance requirements of the intended application, not just the total oxide percentage.
Specifying detailed compositional limits prevents field failures and maximizes component lifetime.
Compositional Specification Matrix
| Application | SiO₂ (%) | Al (ppm) | Fe (ppm) | OH (ppm) | Particle Inclusion (pcs/cm³) |
|---|---|---|---|---|---|
| Semiconductor | >99.995 | <1 | <0.1 | <1 | <0.1 |
| UV Optics | >99.99 | <5 | <0.5 | <10 | <1 |
| IR Optics | >99.99 | <5 | <0.5 | <1 | <1 |
| Furnace Tubes | >99.95 | <10 | <0.5 | <10 | <5 |
| Chemical Processing | >99.95 | <10 | <1 | <50 | <5 |
How Do You Verify Chemical Purity Standards for Critical Applications?
Verification of chemical purity is essential to ensure compliance with application requirements.
Best practices include requesting supplier certificates of analysis (COA), batch traceability, and independent laboratory testing for metallic impurities and OH content. For high-value applications, incoming inspection protocols should include both chemical and physical property verification.
Documenting all verification steps supports traceability and continuous quality improvement.
Purity Verification Protocols
| Verification Step | Method/Tool | Acceptance Criteria |
|---|---|---|
| Supplier COA Review | Document inspection | Meets specified impurity limits |
| Batch Traceability | Lot/batch number | Full traceability to raw material |
| ICP-MS Analysis | Lab testing | Al <10ppm, Fe <0.5ppm, Na+K <5ppm |
| FTIR for OH Content | Spectroscopy | OH < specified ppm |
| Particle Inspection | Microscopy, laser scanning | Inclusion count < specified limit |
Which Analytical Methods Confirm Composition Requirements Accurately?
Accurate compositional analysis is achieved through advanced analytical techniques.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is the gold standard for detecting metallic impurities at sub-ppm levels. Fourier Transform Infrared Spectroscopy (FTIR) is used to quantify OH content. Additional methods include X-ray fluorescence (XRF) for elemental analysis and laser scattering for particle inclusions.
Selecting the appropriate analytical method ensures reliable compliance with compositional specifications.
Analytical Methods for Quartz Glass Composition
| Method | Target Parameter | Detection Limit | Typical Use Case |
|---|---|---|---|
| ICP-MS | Metallic impurities | <0.01 ppm | Al, Fe, Na, K, trace metals |
| FTIR | Hydroxyl (OH) content | <0.1 ppm | OH quantification |
| XRF | Elemental composition | ~1 ppm | Routine screening |
| Laser Scattering | Particle inclusions | <0.1 pcs/cm³ | Inclusion count |
Decision Framework for Composition-Based Quartz Glass Selection
A systematic approach to compositional selection ensures optimal performance and risk mitigation.
The following checklist guides engineers and procurement teams through the critical decision points for specifying quartz glass composition.
Composition Selection Checklist
| Step | Key Question | Recommended Action if "Yes" |
|---|---|---|
| 1 | Is the application high-temperature (>1000°C)? | Specify Al <10ppm, Fe <0.5ppm, OH <10ppm |
| 2 | Is UV/IR transmission critical? | Require Fe <0.5ppm, OH <1ppm (IR) |
| 3 | Is contamination control essential? | Select synthetic quartz, ultra-high purity |
| 4 | Are field failures costly? | Request ICP-MS/FTIR analysis, batch trace |
| 5 | Is custom geometry or tight tolerance required? | Engage suppliers with advanced QC |
Conclusion
Quartz glass composition—especially impurity control—directly determines its suitability for advanced, high-value applications.
Navigating the complexities of compositional selection is a critical engineering challenge. Leverage our factory-direct supply, advanced analytical verification, and engineering support—backed by 20+ years of experience—to ensure your quartz glass meets the strictest purity standards. Contact us for expert consultation and tailored solutions.
FAQ (Frequently Asked Questions)
What is the difference between 99.9% and 99.995% SiO₂ quartz glass?
The higher grade (99.995%) contains significantly lower metallic and OH impurities, resulting in better optical, thermal, and chemical performance for demanding applications.
How can I verify the metallic impurity content in a batch of quartz glass?
Request an ICP-MS analysis report from your supplier and confirm that individual impurity levels (Al, Fe, Na, K) meet your application’s specifications.
What are the risks of using natural quartz glass for semiconductor or UV optics?
Natural quartz typically contains higher metallic and OH impurities, which can cause devitrification, reduced transmission, and contamination—leading to process failures in sensitive environments.
Which analytical method is best for confirming OH content in quartz glass?
Fourier Transform Infrared Spectroscopy (FTIR) is the preferred method for accurately quantifying hydroxyl group concentrations in quartz glass.