
Quartz tubes are produced using high-purity silicon dioxide, also called fused quartz. Purity plays a key role in their performance, especially in industrial settings.
Higher purity SiO₂ increases resistance to harsh chemicals and high temperatures.
Trace impurities, like metals, can decrease chemical stability and make tubes less durable.
Key Takeaways
Quartz tubes are made from high-purity silicon dioxide, which enhances their resistance to chemicals and high temperatures.
The molecular structure of silicon dioxide in quartz tubes is amorphous, providing improved thermal shock resistance and optical clarity.
Selecting the right purity grade of quartz tubes is crucial for performance; higher purity leads to better durability and reliability in demanding applications.
What Is the Primary Chemical Composition of Quartz Tubes?
Quartz tubes stand out because of their nearly pure silicon dioxide content. Their unique structure and high purity levels set them apart from ordinary glass. Understanding their composition helps explain why they perform so well in demanding environments.
Silicon Dioxide Molecular Structure: Tetrahedral Si-O-Si Bond Network
Silicon dioxide forms the backbone of quartz tubes. Each silicon atom bonds to four oxygen atoms, creating a strong tetrahedral network. This arrangement gives the material its remarkable stability.
The molecular structure of silicon dioxide in quartz tubes is amorphous, meaning it lacks a regular, repeating pattern. Unlike crystalline quartz, fused quartz has a disorderly arrangement of atoms. This non-crystalline form results in different physical and chemical properties, such as improved resistance to thermal shock and chemicals. The table below highlights the differences between crystalline and amorphous forms:
Form of Silica | Structure Type | Characteristics |
|---|---|---|
Quartz | Crystalline | Ordered, three-dimensional network |
Fused Quartz | Amorphous | Disordered, glass-like structure |
The amorphous network in fused quartz tubes prevents the formation of weak points, making them ideal for high-temperature and corrosive applications. This structure also allows for high optical clarity and low thermal expansion.
Purity Grades: Industrial (99.9%), Optical (99.98%), Ultra-High-Purity (99.995%+)
Quartz tubes come in several purity grades, each designed for specific uses. The main grades include industrial, optical, and ultra-high-purity, with silicon dioxide content ranging from 99.9% to over 99.995%. Higher purity means fewer impurities, which leads to better performance.
Industrial-grade quartz tubes (99.9% SiO₂) work well for general high-temperature processes. Optical grades (99.98% SiO₂) offer enhanced clarity and are used in scientific and semiconductor industries. Ultra-high-purity tubes (99.995%+ SiO₂) provide the best optical and mechanical properties, making them essential for advanced electronics and photonics. The table below summarizes the key features of each grade:
Purity Grade | Optical Properties | Mechanical Properties |
|---|---|---|
Industrial (99.9%) | Good transparency | High thermal stability |
Optical (99.98%) | Superior clarity | Improved shock resistance |
Ultra-High (99.995%+) | Exceptional optical clarity | Maximum strength and durability |
Higher purity grades reduce the risk of contamination and improve the lifespan of quartz tubes in harsh environments. Selecting the right grade ensures reliable performance for each application.
Trace Impurity Profile: Aluminum, Titanium, Alkali Metals, and Iron Content
Trace impurities in quartz tubes can affect their properties. Common impurities include aluminum, titanium, alkali metals (like sodium and potassium), and iron. These elements usually appear in very small amounts, measured in micrograms per gram.
Even small amounts of these impurities can influence the chemical and optical behavior of quartz tubes. For example, aluminum and titanium can affect UV transmission, while alkali metals may speed up devitrification, reducing mechanical strength. Iron content can cause slight coloration, which may impact optical clarity. The table below shows typical impurity levels:
Trace Impurity | Typical Concentration Range (μg/g) |
|---|---|
Aluminum | Up to several thousand |
Lithium | Often found with aluminum |
Potassium | Often found with aluminum |
Sodium | Often found with aluminum |
Boron | Possible presence |
Phosphorus | Possible presence |
Key Points on Impurities:
Trace elements can impact optical and mechanical properties.
Lower impurity levels mean better performance and longer service life.
Careful control of impurities is essential for high-purity quartz tubes.
What Raw Materials Are Used to Manufacture Quartz Tubes?

Manufacturers select raw materials with great care to ensure the quality and performance of quartz tubes. The choice between natural and synthetic sources affects both purity and cost. Each step in the purification process helps achieve the high standards required for advanced applications.
Natural Quartz Crystals: Spruce Pine and High-Purity Mining Sources
Natural quartz crystals serve as a primary source for many quartz tubes. Mining regions such as Spruce Pine in the United States, Brazil, Africa, and India supply high-purity quartz with SiO₂ content above 99.9%. These crystals undergo careful selection to meet strict industry requirements.
Producers crush, wash, and sort the crystals to remove visible impurities. They often use magnetic separation to eliminate iron and other metals. The resulting material supports the production of tubes with excellent thermal stability and chemical resistance. High-purity quartz from these sources finds use in high-end products across various industries.
Key Points:
Natural quartz crystals offer high SiO₂ purity (over 99.9%).
Major mining regions include the United States, Brazil, Africa, and India.
Careful selection and processing ensure suitability for demanding applications.
Synthetic Silica: Silicon Tetrachloride CVD Processes for Ultra-High Purity
Synthetic silica, produced through chemical vapor deposition (CVD) using silicon tetrachloride, achieves even higher purity than natural quartz. This process creates fused silica with minimal impurities, making it ideal for applications that demand the highest optical clarity and chemical resistance.
The CVD method involves burning silicon tetrachloride in an oxygen-hydrogen flame, forming pure silica particles that fuse into glass. This synthetic route results in SiO₂ purity levels exceeding 99.995%. However, the cost of synthetic silica is five to ten times higher than natural quartz, which limits its use to specialized fields like semiconductor manufacturing.
Material Type | Typical SiO₂ Purity | Relative Cost | Best Use |
|---|---|---|---|
Natural Quartz Crystal | >99.9% | Lower | General and industrial |
Synthetic Silica (CVD) | >99.995% | Much higher | Semiconductor, optics |
Raw Material Purification: Acid Leaching, Flotation, and Thermal Treatment
Purification processes play a crucial role in preparing raw materials for quartz tubes. Acid leaching removes metallic impurities by soaking quartz in strong acids. If the material does not meet purity standards, manufacturers repeat this step to achieve the desired result.
Flotation separates quartz from other minerals by exploiting differences in surface properties. This method effectively removes feldspar and silicate minerals. Calcination, or thermal treatment, heats quartz to high temperatures, causing cracks that expose hidden impurities for easier removal. Studies show that combining reverse-flotation with acid leaching can raise SiO₂ purity to 99.9980%.
Purification Highlights:
Acid leaching and flotation remove metallic and silicate impurities.
Thermal treatment exposes inclusions for further purification.
Combined methods can achieve ultra-high SiO₂ purity.
How Do Manufacturing Processes Transform Raw Materials Into Quartz Tubes?

Manufacturers use advanced processes to convert raw quartz into high-performance tubes. Each method influences the purity, durability, and application of the final product. Understanding these steps helps explain why quartz tubes excel in demanding environments.
Electrical Fusion: Controlled-Atmosphere Continuous Melting Process
Engineers begin by washing and drying natural quartz to remove contaminants. They crush and mill the quartz to prepare it for fusion. The fusion process uses electric heating to break silicon-oxygen bonds, transforming the material into a glassy structure.
Continuous electrical fusion operates in a controlled atmosphere, which prevents contamination and maintains low hydroxyl content. This method produces quartz tubes with high purity and consistent dimensions. The process supports both continuous and batch modes, allowing for flexible production.
Key Steps in Electrical Fusion:
Washing and Drying: Removes dirt and moisture.
Comminution: Reduces quartz size for fusion.
Fusion: Converts quartz to glass using electric heating.
This process ensures reliable performance in applications that require high chemical resistance and thermal stability.
Flame Fusion: Oxyhydrogen Combustion and High-Temperature Deposition
Flame fusion uses an oxyhydrogen flame that reaches temperatures up to 2800 degrees. This intense heat melts quartz efficiently, allowing for effective tube sealing and smooth surfaces. The process reduces porosity and improves surface quality, which enhances chemical resistance.
Flame polishing eliminates micropores and prevents the "orange peel" effect. The resulting surface resists hydrofluoric acid and supports thinner seals for vacuum applications. These improvements increase the structural integrity and purity of the tubes.
Process Feature | Benefit |
|---|---|
High Temperature | Effective melting and sealing |
Flame Polishing | Smooth surface, increased resistance |
Thin Seals | Improved vacuum performance |
Manufacturers choose flame fusion for applications that require superior surface quality and chemical durability.
Tube Forming and Post-Fusion Processing: Annealing, Cutting, End Finishing
After fusion, tube forming shapes the molten quartz into precise dimensions. Annealing slowly cools the tubes to relieve internal stress and prevent cracking. Cutting and end finishing create the final product, ready for use.
Quality control follows international standards such as ISO 12123 and ASTM C100. Detailed test reports and batch traceability ensure consistent quality and supplier reliability. Certification and audits verify that each batch meets strict requirements.
Quality Assurance Highlights:
International Standards: Ensure traceability and consistency.
Testing Methods: Verify purity and durability.
Supplier Audits: Maintain high standards.
These steps guarantee that quartz tubes meet the needs of industries where reliability and performance are critical.
What Quality Standards Validate Quartz Tube Material Composition?
Quality standards play a vital role in ensuring the reliability and performance of quartz tubes. These standards guide manufacturers in verifying chemical composition, physical properties, and measurement accuracy. By following strict protocols, industries can trust that each tube meets the demands of advanced applications.
ISO 12123 Chemical Composition Analysis: ICP-OES and GDMS Testing
ISO 12123 sets the benchmark for chemical analysis of quartz materials. Laboratories use advanced techniques like Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and Glow Discharge Mass Spectrometry (GDMS) to detect even the smallest impurities. These methods help manufacturers confirm that quartz tubes meet strict purity requirements.
ICP-OES measures metallic impurities with detection limits below 0.1 parts per million, making it suitable for furnace and chemical processing tubes. GDMS offers even greater sensitivity, detecting trace metals down to 0.01 parts per million, which is essential for semiconductor and optical applications. The table below summarizes the main features of these methods:
Method | Target Parameter | Detection Limit | Typical Use Case |
|---|---|---|---|
ICP-OES | Metallic Impurities | <0.1 ppm | Furnace, chemical tubes |
GDMS | Trace Metals | <0.01 ppm | Semiconductor, optics |
Key Takeaways:
High-sensitivity analysis ensures consistent purity.
Advanced testing methods support demanding industries.
ISO 12123 compliance builds confidence in material quality.
Physical Property Verification: Density, Thermal Expansion, Optical Transmission
Physical property testing confirms that quartz tubes will perform as expected in real-world conditions. Laboratories measure density, thermal expansion, and optical transmission to verify that each batch meets industry standards. These properties affect how tubes handle heat, pressure, and light.
Density measurements should reach 2.2 g/cm³, while the coefficient of thermal expansion typically falls near 5.5 × 10^-7 cm/cm·°C between 20°C and 320°C. Optical transmission tests check that tubes allow light to pass from ultraviolet to infrared wavelengths, which is critical for scientific and industrial uses. The following table highlights typical values for these properties:
Property | Typical Values |
|---|---|
Density | 2.2 × 10³ kg/m³ |
Coefficient of Thermal Expansion | 5.5 × 10^-7 cm/cm·°C (20°C-320°C) |
Optical Transmission | Ultraviolet to infrared |
Physical Property Summary:
Consistent density and expansion rates prevent failure.
High optical transmission supports advanced optics.
Testing ensures tubes meet application needs.
ISO/IEC 17025 Laboratory Accreditation and Measurement Traceability
Accredited laboratories provide confidence in test results. ISO/IEC 17025 accreditation ensures that labs follow strict procedures and use calibrated equipment. This standard requires traceability to national measurement institutes, such as the National Institute of Standards and Technology (NIST).
Traceability means every measurement links back to a recognized standard. This process guarantees that results remain accurate and comparable worldwide. The table below outlines the key aspects of ISO/IEC 17025 accreditation:
Accreditation Standard | Traceability Source | Description |
|---|---|---|
National Institute of Standards and Technology (NIST) | Ensures calibration standards for quartz tube testing are traceable and meet international standards. |
Accreditation Summary:
ISO/IEC 17025 ensures reliable, repeatable measurements.
Traceability links results to trusted standards.
Accredited labs support global quality assurance.
How Should Engineers Specify Quartz Tube Material Requirements for Procurement?
Engineers must define clear material requirements when procuring quartz tubes for high-performance applications. These requirements help ensure that every tube meets the necessary standards for purity, durability, and traceability. By specifying detailed parameters, engineers can avoid unexpected failures and maintain consistent quality.
Defining Multi-Parameter Purity Requirements vs. Single Total Purity Limits
Engineers often choose multi-parameter purity requirements instead of relying on a single total purity limit. This approach allows them to control individual impurity levels, which can affect tube performance in critical environments. For example, limiting aluminum, calcium, iron, sodium, and potassium ensures better optical and mechanical properties.
A summary table shows recommended values for high-performance quartz tubes:
Property | Value |
|---|---|
SiO₂ | 99.99% |
Maximum Temperature | 1250°C |
Corrosion Resistance | Excellent |
Thermal Expansion Coefficient | 5.5×10⁻⁷ cm/cm·°C |
Light Transmittance | Excellent |
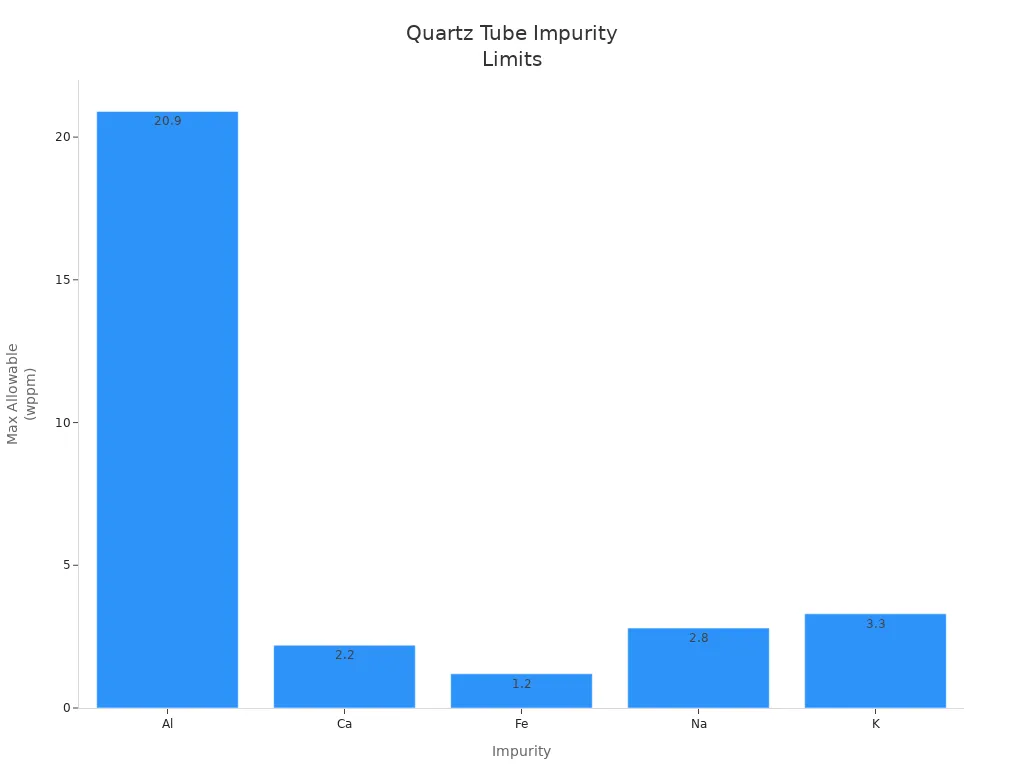
Impurity | Maximum Allowable (wppm) |
|---|---|
Al | 20.9 |
Ca | 2.2 |
Fe | 1.2 |
Na | 2.8 |
K | 3.3 |
Multi-parameter purity control improves reliability and extends service life.
Specifying Analytical Methods, Detection Limits, and Measurement Uncertainty
Engineers must specify analytical methods to verify purity and impurity levels. Common techniques include ICP-OES and GDMS, which detect trace metals at very low concentrations. These methods provide accurate results and help confirm that tubes meet strict specifications.
Detection limits and measurement uncertainty play a key role in quality assurance. For example, GDMS can detect iron at levels below 1.2 wppm, while ICP-OES measures sodium and potassium with high precision. Engineers should request test reports that list individual impurity concentrations and include method details.
Summary of Analytical Specification:
Method | Detection Limit | Application |
|---|---|---|
ICP-OES | <0.1 ppm | General purity checks |
GDMS | <0.01 ppm | Semiconductor, optics |
Specifying methods and limits ensures consistent quality and supports application needs.
Verification Frequency, Batch Definition, and Traceability Requirements
Verification frequency and batch definition help maintain quality across production lots. Engineers may require 100% batch testing for critical applications or statistical sampling for general use. Clear batch definitions, such as production date or furnace heat number, support traceability.
Batch traceability ensures compliance with specifications and enhances safety. It also supports material validation, which is vital for sectors like healthcare and semiconductors. Suppliers who document manufacturing controls and demonstrate consistent performance can meet stringent procurement requirements.
Key Points for Traceability:
Batch traceability supports quality assurance and safety.
Defined batch testing reduces risk of non-conformance.
Documented controls enable supplier qualification.
Quartz tubes deliver outstanding performance because manufacturers use nearly pure silicon dioxide and control impurities with advanced processes. The table below highlights key differences between quartz tubes and ordinary glass:
Factor | Quartz Tubes | Glass Tubes |
|---|---|---|
Material Composition | 99.99% pure silica | Silica mixed with other compounds |
Thermal Properties | Withstand up to 1200°C (2192°F) | Withstand up to 500°C (932°F) |
Chemical Resistance | Highly resistant to acids and bases | Less resistant to strong chemicals |
Applications | Used in semiconductors, optics, etc. | Common in household items, jewelry |
Cost and Manufacturing | More expensive due to high purity | Less expensive and easier to manufacture |
Engineers should always consider purity, manufacturing methods, and certification when selecting quartz tubes for critical applications.
FAQ
What makes quartz tubes different from regular glass tubes?
Higher purity: Quartz tubes contain over 99.9% SiO₂.
Better heat resistance: They withstand temperatures up to 1200°C.
Superior chemical durability: They resist acids and bases.
How do manufacturers test quartz tube purity?
Laboratories use ICP-OES and GDMS methods. These tests detect impurities below 0.1 ppm, ensuring quartz tubes meet strict industry standards.
Where do manufacturers source high-purity quartz for tubes?
Source | Typical SiO₂ Purity |
|---|---|
Spruce Pine, USA | 99.9%+ |
Synthetic silica (CVD) | 99.995%+ |
Manufacturers select sources based on required purity and application.





