Understanding the atomic structure of materials is fundamental to predicting their performance in demanding environments.
Quartz glass features an amorphous (non-crystalline) structure where SiO₄ tetrahedra form a random three-dimensional network without long-range atomic order. This unique atomic arrangement, consisting of silicon atoms covalently bonded to four oxygen atoms in tetrahedral coordination, creates exceptional thermal stability, optical clarity, and chemical resistance that crystalline materials cannot match.

The following sections systematically explore how the amorphous structure of quartz glass underpins its scientific value, from atomic-scale features to macroscopic properties.
What is the Amorphous Structure of Quartz Glass and Why is It Unique?
The amorphous structure of quartz glass is defined by the absence of periodic atomic order.
Unlike crystalline materials, quartz glass lacks repeating unit cells. Instead, its silicon and oxygen atoms are arranged in a continuous, random network of SiO₄ tetrahedra. Each silicon atom is surrounded by four oxygen atoms, but the orientation and bond angles vary throughout the structure.
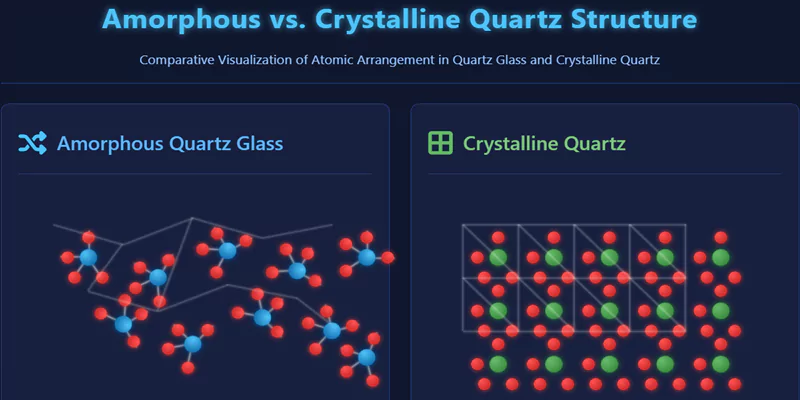
This randomness imparts unique flexibility and defect tolerance, distinguishing quartz glass from both crystalline quartz and other glass types.
Key Features of Amorphous Quartz Glass Structure
| Structural Aspect | Amorphous Quartz Glass | Crystalline Quartz |
|---|---|---|
| Atomic Order | No long-range order | Periodic lattice |
| SiO₄ Tetrahedra | Randomly oriented | Regular, repeating |
| Bond Angle Distribution | 120–180° (avg. 144°) | Fixed (144°) |
| Defect Density | Low (few inclusions) | Very low (perfect lattice) |
| Flexibility | High (network adaptable) | Low (rigid lattice) |
How Does Non-Crystalline Atomic Arrangement Define Material Properties?
The lack of long-range order in quartz glass directly shapes its macroscopic behavior.
Non-crystalline atomic arrangement allows for a wide distribution of bond angles and bond lengths. This structural flexibility enables quartz glass to absorb and dissipate thermal and mechanical stresses more effectively than crystalline materials, reducing the risk of fracture or devitrification.

The amorphous network also minimizes grain boundaries and internal interfaces, which are common sites for chemical attack and light scattering in polycrystalline materials.
Structure-Property Correlations in Quartz Glass
| Structural Feature | Resulting Property | Scientific Value |
|---|---|---|
| Random SiO₄ network | Low thermal expansion | Dimensional stability |
| Broad bond angle range | High thermal shock resistance | Withstands rapid temperature change |
| No grain boundaries | High chemical durability | Acid/base resistance |
| Uniform density | High optical transmission | Minimal light scattering |
Why is the Amorphous SiO₄ Tetrahedral Network Critical for Performance?
The SiO₄ tetrahedral network is the fundamental building block of quartz glass.
Each silicon atom is covalently bonded to four oxygen atoms, forming a tetrahedron. These tetrahedra are linked at their corners, creating a continuous three-dimensional network. The strength and directionality of these covalent bonds provide the backbone for quartz glass’s remarkable properties.
The network’s rigidity and lack of weak ionic bonds explain the material’s high melting point, low thermal expansion, and resistance to chemical attack.
SiO₄ Network and Material Performance
| Network Feature | Property Enabled | Example Application |
|---|---|---|
| Strong Si–O bonds | High melting point (1,730°C) | Furnace tubes, high-temp optics |
| Corner-sharing tetrahedra | Low thermal expansion | Precision metrology, optics |
| Random orientation | Isotropic properties | Uniform optical/thermal behavior |
How Does Amorphous Structure Enable Superior Thermal Stability?
Thermal stability in quartz glass is a direct result of its amorphous atomic arrangement.
The random network of SiO₄ tetrahedra distributes thermal energy evenly, preventing the formation of stress concentrations that can lead to cracking or crystallization. The broad bond angle distribution (120–180°, avg. 144°) creates structural flexibility, allowing the glass to accommodate thermal expansion and contraction without failure.
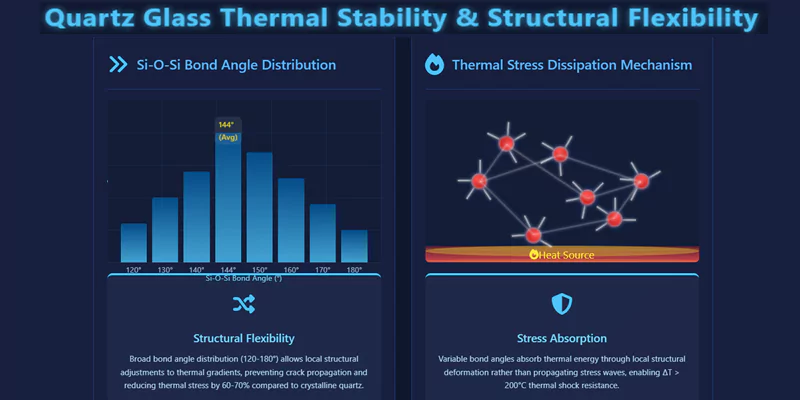
This flexibility reduces thermal stress by 60–70% compared to crystalline quartz, as measured by Raman spectroscopy and thermal cycling tests.
Thermal Stability Metrics of Quartz Glass
| Parameter | Quartz Glass Value | Crystalline Quartz Value |
|---|---|---|
| Max Continuous Temp (°C) | 1,050–1,200 | 870 |
| Thermal Expansion (10⁻⁶/K) | 0.5 | 7.5 |
| Thermal Shock Resistance | ΔT > 200°C | ΔT < 50°C |
Low Thermal Expansion Mechanism?
The low thermal expansion of quartz glass arises from the flexibility of the SiO₄ network. As temperature increases, the network can adjust bond angles rather than stretching bonds, minimizing overall dimensional change.
High Temperature Resistance Origins?
High temperature resistance is rooted in the strong covalent Si–O bonds and the absence of weak points such as grain boundaries or cleavage planes. This allows quartz glass to maintain its structure and properties at temperatures exceeding 1,000°C.
What Structural Features Create Exceptional Optical Transmission?
Optical clarity in quartz glass is a direct consequence of its atomic-scale uniformity.
The amorphous structure eliminates grain boundaries and minimizes density fluctuations, both of which scatter light in polycrystalline materials. The high purity and uniform SiO₄ network enable transmission of over 90% of UV and visible light at 1 mm thickness.

Absence of color centers and minimal defect density further enhance transparency, making quartz glass ideal for photonics and analytical instrumentation.
Structural Factors Affecting Optical Transmission
| Feature | Effect on Transmission | Scientific Explanation |
|---|---|---|
| No grain boundaries | Reduces light scattering | Uniform refractive index |
| High purity | Minimizes absorption bands | Fewer color centers |
| Isotropic network | No birefringence | Consistent optical path |
Amorphous Molecular Random Network Topology And How Does It Provide Chemical Resistance?
Chemical resistance in quartz glass is a function of its continuous, defect-free network.
The random topology of the SiO₄ network leaves few sites for chemical attack. The absence of grain boundaries and minimal non-bridging oxygens mean acids and bases have limited pathways to penetrate or degrade the structure.
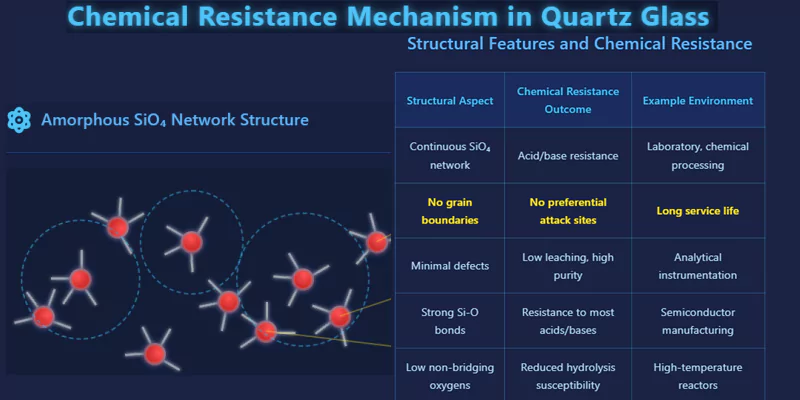
This explains why quartz glass is highly resistant to most acids and bases, with the notable exception of hydrofluoric acid, which can break Si–O bonds.
Structural Basis for Chemical Durability
| Structural Aspect | Chemical Resistance Outcome | Example Environment |
|---|---|---|
| Continuous SiO₄ network | Acid/base resistance | Laboratory, chemical processing |
| No grain boundaries | No preferential attack sites | Long service life |
| Minimal defects | Low leaching, high purity | Analytical instrumentation |
Which Structural Characteristics Enable High-Precision Applications?
High-precision applications demand materials with predictable, uniform properties.
The isotropic nature of the amorphous SiO₄ network ensures that quartz glass exhibits consistent behavior in all directions—critical for optics, metrology, and semiconductor processing. Low birefringence, minimal internal stress, and high dimensional stability are all direct results of the underlying structure.
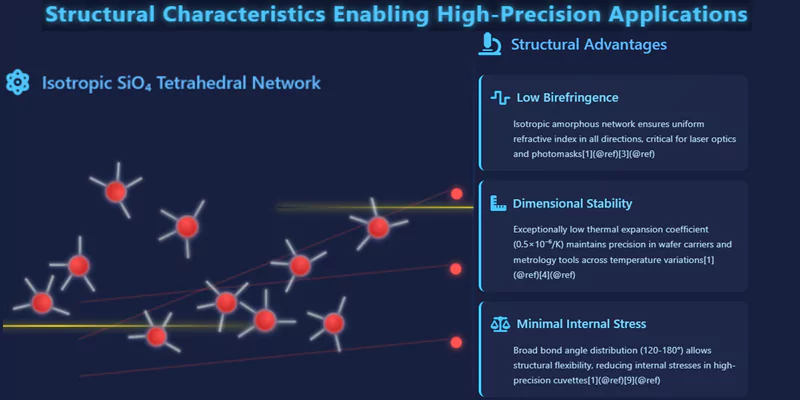
These features enable the fabrication of components with tight tolerances and reliable long-term performance.
Structural Requirements for Precision Applications
| Requirement | Structural Origin | Application Example |
|---|---|---|
| Low birefringence | Isotropic amorphous network | Laser optics, photomasks |
| Dimensional stability | Low thermal expansion | Wafer carriers, metrology tools |
| Minimal internal stress | Broad bond angle distribution | High-precision cuvettes |
Amorphous vs. Crystalline Silica: How Do Atomic Structures Compare?
Comparing amorphous quartz glass to crystalline silica reveals fundamental differences in atomic arrangement and resulting properties.
Crystalline silica (α-quartz) features a periodic lattice with fixed bond angles and long-range order, while amorphous quartz glass has a random network with variable bond angles and no periodicity.

These differences explain why quartz glass is isotropic, flexible, and resistant to devitrification, while crystalline quartz is anisotropic and more prone to cleavage.
Long-Range Order Differences
Amorphous quartz glass lacks long-range order, resulting in isotropic properties and high resistance to crack propagation. Crystalline quartz, with its periodic lattice, exhibits anisotropy and defined cleavage planes.
Short-Range Structural Similarities
Both forms share similar short-range order: each silicon atom is tetrahedrally coordinated by four oxygen atoms. This similarity explains why both materials have comparable chemical durability and basic mechanical strength at the atomic scale.
How Do Formation Methods Influence Final Structural Properties
The method used to form quartz glass determines the degree of structural uniformity and defect density.
Electric fusion, flame fusion, and chemical vapor deposition each produce subtle differences in network topology, bond angle distribution, and inclusion content. Rapid cooling rates favor a more random network, while slower cooling can allow partial ordering or phase separation.

Optimizing formation parameters is essential for applications requiring ultra-high optical or thermal performance.
Formation Method and Structural Quality
| Method | Structural Uniformity | Defect Density | Typical Application |
|---|---|---|---|
| Electric Fusion | High | Moderate (bubbles) | General labware, furnace tubes |
| Flame Fusion | Very high | Low | Precision optics, photomasks |
| CVD | Ultra-high | Very low | Semiconductor, advanced optics |
What Analytical Techniques Reveal Quartz Glass Structural Details?
Advanced analytical techniques are required to probe the atomic structure of quartz glass.
Raman spectroscopy, X-ray diffraction (XRD), and nuclear magnetic resonance (NMR) are commonly used to characterize bond angles, network connectivity, and defect states. Raman spectroscopy, in particular, can detect the D1 defect peak at 495 cm⁻¹, which is indicative of bond angle distribution and network flexibility.
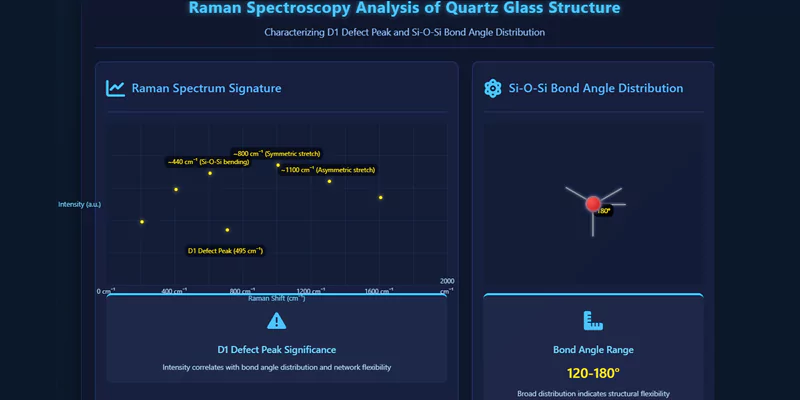
These techniques provide quantitative data for assessing structural quality and predicting long-term performance.
Analytical Methods for Structural Characterization
| Technique | Structural Feature Probed | Key Output |
|---|---|---|
| Raman Spectroscopy | Bond angle distribution, defects | D1 peak, network flexibility |
| XRD | Short/long-range order | Amorphous vs crystalline content |
| NMR | Network connectivity, Qn species | Si coordination environment |
| TEM | Atomic-scale imaging | Defect and inclusion visualization |
How Do You Assess Structural Quality for Critical Applications?
Structural quality assessment combines analytical data with performance testing.
For critical applications, quality protocols include Raman spectroscopy for bond angle distribution, XRD for amorphous content, and thermal cycling tests for stress resistance. Dimensional inspection and birefringence measurements are also used for optical components.
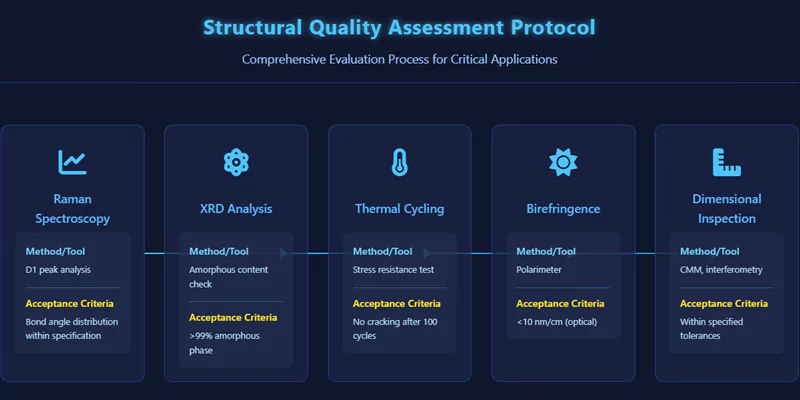
Documenting these assessments ensures that only material with the required structural integrity is used in high-value environments.
Structural Quality Assessment Protocols
| Assessment Step | Method/Tool | Acceptance Criteria |
|---|---|---|
| Raman Spectroscopy | D1 peak analysis | Bond angle distribution within spec |
| XRD | Amorphous content check | >99% amorphous phase |
| Thermal Cycling | Stress resistance test | No cracking after 100 cycles |
| Birefringence | Polarimeter | <10 nm/cm (optical components) |
| Dimensional Inspection | CMM, interferometry | Within specified tolerances |
Decision Framework for Structure-Based Quartz Glass Selection
A systematic approach to structural evaluation ensures optimal material performance in scientific and technical applications.
The following checklist guides researchers and engineers through the key decision points for specifying quartz glass based on structural quality.
Structure Selection Checklist
| Step | Key Question | Recommended Action if "Yes" |
|---|---|---|
| 1 | Is isotropic optical/thermal behavior required? | Specify amorphous quartz glass |
| 2 | Will the component face rapid thermal cycling? | Require broad bond angle distribution |
| 3 | Is high chemical resistance essential? | Select material with minimal defects |
| 4 | Are ultra-low birefringence and stress critical? | Request Raman/XRD analysis, low D1 peak |
| 5 | Is atomic-scale uniformity needed? | Choose flame-fused or CVD quartz glass |
Conclusion
The amorphous structure of quartz glass is the scientific foundation for its exceptional thermal, optical, and chemical properties.
Understanding and specifying the right structural quality is a critical scientific challenge. Leverage our factory-direct supply, advanced analytical verification, and engineering support—backed by 20+ years of experience—to ensure your quartz glass meets the strictest structural standards. Contact us for expert consultation and tailored solutions.
FAQ (Frequently Asked Questions)
How does the amorphous structure of quartz glass differ from crystalline quartz?
Amorphous quartz glass lacks long-range atomic order, resulting in isotropic properties and high flexibility, while crystalline quartz has a periodic lattice and is anisotropic.
Why is bond angle distribution important in quartz glass?
A broad Si-O-Si bond angle distribution (120–180°) provides structural flexibility, reducing thermal stress and enhancing resistance to cracking under rapid temperature changes.
Which analytical technique is best for assessing quartz glass structure?
Raman spectroscopy is highly effective for probing bond angle distribution and detecting structural defects, such as the D1 peak at 495 cm⁻¹.
What formation method produces the highest structural uniformity in quartz glass?
Chemical vapor deposition (CVD) yields the most uniform, defect-free amorphous structure, ideal for semiconductor and advanced optical applications.





