Selecting materials for high-performance engineering often hinges on understanding their thermal properties. Quartz glass stands out for its unique combination of low thermal conductivity and exceptional stability.
Quartz glass exhibits a thermal conductivity of 1.38 W/m·K at 25°C, making it an exceptional thermal insulator compared to metals while maintaining superior optical and chemical properties. This unique combination enables critical applications in semiconductor processing, high-precision optics, and high-temperature environments where thermal stability is paramount.
Understanding the thermal conductivity of quartz glass is essential for engineers and designers aiming to optimize performance in demanding environments. The following sections provide a comprehensive analysis, from fundamental principles to practical application guidance.
What Is Thermal Conductivity and Why Is It Critical?
Thermal management is a central engineering challenge in advanced manufacturing and electronics. The ability of a material to conduct heat directly impacts system reliability and efficiency.
Thermal conductivity measures how efficiently a material transfers heat. High conductivity means rapid heat flow, while low conductivity indicates strong insulation. For quartz glass, its low thermal conductivity is a defining property that shapes its use in high-tech industries.
Thermal conductivity (λ) is defined as the amount of heat (in watts) passing through a material of 1 meter thickness per square meter per degree Kelvin temperature difference. This property is crucial in applications where temperature gradients must be controlled, such as semiconductor wafer processing or optical system alignment. Materials with low thermal conductivity, like quartz glass, help maintain thermal stability, reduce thermal shock risk, and protect sensitive components from rapid temperature changes.
Key Thermal Conductivity Concepts
| Property | Description |
|---|---|
| Definition | Heat transferred per unit thickness, area, and temperature difference (W/m·K) |
| High Conductivity | Rapid heat dissipation (e.g., metals like copper, aluminum) |
| Low Conductivity | Thermal insulation (e.g., quartz glass, ceramics) |
| Engineering Importance | Impacts thermal management, energy efficiency, and material selection in critical systems |
What Is the Exact Thermal Conductivity Value of Quartz Glass?
Precise data is essential for engineering calculations. Quartz glass (fused silica) typically has a thermal conductivity of 1.38 W/m·K at 25°C, but this value can vary with temperature and purity.
At room temperature (25°C), the thermal conductivity of quartz glass is approximately 1.38 W/m·K, which is significantly lower than most metals and many ceramics.
Quartz glass’s low thermal conductivity is a result of its amorphous structure, which impedes phonon transport. Unlike crystalline materials, the disordered atomic arrangement in quartz glass scatters heat-carrying vibrations, resulting in superior insulation. This property is stable across a wide temperature range, making quartz glass ideal for environments where both thermal and chemical stability are required.
Thermal Conductivity of Quartz Glass: Reference Values
| Temperature (°C) | Thermal Conductivity (W/m·K) | Context/Notes |
|---|---|---|
| 25 | 1.38 | Standard reference value |
| 100 | 1.40–1.45 | Slight increase with temperature |
| 500 | 1.60–1.70 | Gradual rise, remains low compared to metals |
| 1000 | 1.90–2.10 | Still an effective insulator |
How Does Temperature Affect These Values?
Thermal conductivity in quartz glass increases gradually as temperature rises, but the change is modest compared to metals. At 1000°C, the value typically reaches around 2.0 W/m·K. This stability ensures predictable performance in high-temperature applications, such as furnace tubes or crucibles.
What Factors Influence Measurement Accuracy?
Measurement accuracy depends on sample purity, surface finish, and the method used (steady-state vs. transient techniques). Impurities and microstructural defects can slightly alter results, but high-purity quartz glass offers consistent, reproducible values.
How Do OH Content and Purity Affect Thermal Performance?
Hydroxyl (OH) groups disrupt the silica network by breaking Si–O–Si bonds, which increases phonon scattering and slightly lowers thermal conductivity.
While this effect is negligible for most industrial uses, it becomes more significant in high-precision environments.
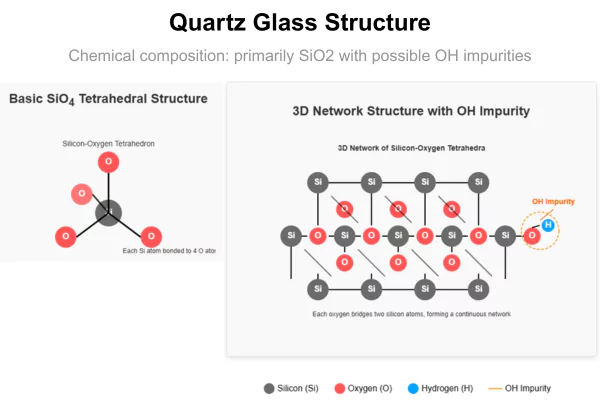
In applications such as semiconductor processing or advanced optics, maintaining low OH content and high material purity is essential to ensure stable and predictable thermal behavior.
Purity and OH Content Impact
| Parameter | Typical Range | Effect on Thermal Conductivity | Application Relevance |
|---|---|---|---|
| OH Content (ppm) | <1 to >1000 | Higher OH = Slightly lower conductivity | Critical for optics, semiconductors |
| Metal Impurities | <1 ppm (high purity) | Minimal effect at low levels | High-purity required for electronics |
| Structural Defects | Varies | Can reduce conductivity | Minimized in quality-controlled production |
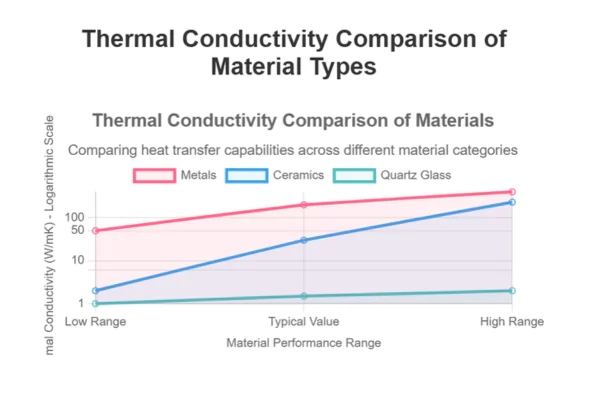
How Does Quartz Glass Compare to Other Materials?
Material selection often involves comparing thermal properties across options. Quartz glass’s low thermal conductivity sets it apart from metals and many ceramics, but how does it compare to sapphire, alumina, and borosilicate glass?
Quartz glass has a much lower thermal conductivity than sapphire and alumina, and is also a better insulator than borosilicate glass.
The differences in thermal conductivity have direct implications for thermal management, insulation, and component longevity. Quartz glass is preferred where thermal insulation and chemical purity are critical, while sapphire and alumina are chosen for applications demanding higher thermal conductivity and mechanical strength.
Comparative Thermal Conductivity
| Material | Thermal Conductivity (W/m·K at 25°C) | Key Application Context |
|---|---|---|
| Quartz Glass | 1.38 | High-temp insulation, optics, semiconductors |
| Sapphire (Al₂O₃) | 25–46 | LEDs, substrates, high-power electronics |
| Alumina (Polycrystal) | 18–35 | Electrical insulators, substrates |
| Borosilicate Glass | 1.1–1.4 | Laboratory glassware, lighting |
| Fused Silica | 1.38 | UV optics, semiconductor processing |
| Soda-Lime Glass | 0.8–1.0 | Windows, containers |
Quartz Glass vs. Sapphire (Al₂O₃)
Sapphire has a much higher thermal conductivity (25–46 W/m·K) compared to quartz glass (1.38 W/m·K).
However, quartz glass offers superior thermal shock resistance, making it more reliable in rapid temperature changes.
Its excellent UV transparency and chemical purity also make it ideal for semiconductor and optical applications.
Quartz Glass vs. Polycrystalline Alumina
Polycrystalline alumina conducts heat better (18–35 W/m·K), but quartz glass maintains dimensional stability under extreme heat.
Quartz glass is less prone to thermal expansion, reducing the risk of cracking or distortion.
This makes it a preferred choice for high-precision environments like wafer processing.
Quartz Glass vs. Borosilicate Glass
Borosilicate glass has a similar thermal conductivity range (1.1–1.4 W/m·K), but quartz glass performs better at higher temperatures.
Quartz glass can withstand more aggressive thermal cycling without degradation.
Its purity and UV transparency also give it an edge in semiconductor and optical systems.
Quartz Glass vs. Fused Silica
Fused silica and quartz glass share the same thermal conductivity (1.38 W/m·K) and similar chemical composition.
However, quartz glass is often manufactured with tighter purity controls for semiconductor-grade applications.
Its consistent performance under thermal stress makes it a trusted material in critical industries.
Quartz Glass vs. Soda-Lime Glass
Soda-lime glass has a lower thermal conductivity (0.8–1.0 W/m·K), but it lacks the thermal stability of quartz glass.
Quartz glass can endure much higher temperatures without softening or deforming.
This makes it far more suitable for demanding environments like high-temperature furnaces and UV systems.
Industries Leveraging Quartz Glass for Thermal Performance
The unique thermal conductivity of quartz glass enables its use in industries where thermal insulation and stability are paramount.
Quartz glass components such as tubes, crucibles, and plates offer a unique combination of thermal insulation, chemical purity, and UV transparency—making them essential in high-precision and high-temperature environments.
![]()
Key industries benefiting from quartz glass’s thermal properties include:
- Semiconductor Manufacturing:In wafer processing, quartz crucibles are used to contain molten silicon due to their high thermal stability and chemical inertness.
- Optical Systems: Quartz plates are critical in optical assemblies where thermal expansion must be minimized to preserve alignment and image clarity. Their low thermal distortion ensures consistent optical performance even under fluctuating temperatures.
- High-Temperature Furnaces: Quartz tubes are widely used in high-temperature furnace applications, where they endure rapid thermal cycling and resist corrosion from aggressive gases. Their transparency to UV also makes them ideal for UV sterilization systems requiring both thermal and optical stability.
Application-Specific Benefits
| Application Area | Quartz Glass Role | Thermal Conductivity Advantage |
|---|---|---|
| Semiconductor Processing | Wafer carriers, furnace tubes, crucibles | Maintains uniform temperature, prevents contamination |
| Optical Instruments | Windows, lenses, plates | Reduces thermal distortion, preserves alignment |
| High-Temp Furnaces | Insulation, protective liners | Withstands thermal shock, ensures stability |
| UV Sterilization | Quartz tubes for UV lamps | Maintains lamp efficiency, resists heat |
What Are the Design Considerations for Engineers?
Engineers must balance thermal, mechanical, and optical requirements when specifying quartz glass components.
Key considerations include operating temperature, thermal gradients, component geometry, and purity requirements.
When designing with quartz glass, engineers should:
- Evaluate maximum and continuous operating temperatures.
- Consider thermal expansion and potential for thermal shock.
- Specify purity and OH content based on application sensitivity.
- Optimize component geometry for uniform heat distribution, and consider using.
- Account for joining methods and compatibility with other materials.
Engineering Design Checklist
| Design Factor | Recommended Practice | Rationale |
|---|---|---|
| Operating Temperature | Specify both max and continuous ratings | Prevents thermal degradation |
| Purity/OH Content | Match to application (e.g., low-OH for optics) | Ensures performance consistency |
| Geometry | Avoid sharp corners, ensure uniform thickness | Reduces thermal stress |
| Joining Methods | Use compatible adhesives or mechanical joints | Maintains integrity at high temperatures |
| Surface Finish | Specify as needed for optical/thermal needs | Minimizes scattering, improves reliability |
Conclusion
Quartz glass’s low thermal conductivity and stability make it indispensable for advanced engineering applications.
FAQ (Frequently Asked Questions)
Q1: Does quartz glass maintain its thermal conductivity at high temperatures?
Yes, while the thermal conductivity of quartz glass increases slightly with temperature, it remains a strong insulator even at 1000°C, ensuring reliable performance in high-temperature environments.
Q2: How does quartz glass’s thermal conductivity compare to metals?
Quartz glass has a much lower thermal conductivity than metals like copper or aluminum, making it ideal for insulation and thermal management where heat transfer must be minimized.
Q3: Is the thermal conductivity of quartz glass affected by UV exposure?
No, UV exposure does not significantly alter the thermal conductivity of quartz glass, which is why it is widely used in UV lamp applications and optical systems.
Q4: Can impurities significantly impact the thermal conductivity of quartz glass?
At typical impurity levels found in high-purity quartz glass, the effect on thermal conductivity is minimal. However, for ultra-sensitive applications, specifying low-OH and high-purity grades is recommended.





