
Comparing Material Properties and Applications
Explore key differences between quartz and glass tubes.
Features | TOQUARTZ Fused Quartz Glass Tubes | Standard Glass Tubes |
|---|---|---|
Material Composition | High-purity silica (99.98%, up to 99.995%) | Silica mixed with other compounds |
Thermal Resistance | Exceptional resistance to high temperatures | Less resistant to extreme conditions |
Chemical Resistance | High resistance to chemical corrosion | Lower resistance to chemical exposure |
Applications | Ideal for semiconductors, optics, and vacuum systems | Suitable for household items and decorative objects |
Purity Level | Ultra-low impurity content (<20 ppm) | Higher impurity levels (500-3000 ppm) |
Service Life | 18-36 months at high temperatures | 3-8 months at high temperatures |
Softening Point | Softens at ~1730°C | Softens at 696-820°C |
Thermal Expansion | Low thermal expansion coefficient | Higher thermal expansion coefficient |
The difference between glass tube and quartz tube centers on their material composition and how this affects performance in demanding environments. Engineers and laboratory professionals often compare these tubes based on factors like temperature resistance, chemical durability, and purity. The table below highlights critical distinctions that influence decision-making in industrial and scientific applications:
Property | Glass Tubes | |
|---|---|---|
Material Composition | High-purity silica (99.99%) | Silica mixed with other compounds |
Thermal Resistance | Exceptional resistance to high temperatures | Less resistant to extreme conditions |
Chemical Resistance | High resistance to chemical corrosion | Lower resistance to chemical exposure |
Applications | Ideal for semiconductors, optics, and vacuum systems | Suitable for household items and decorative objects |
Key Takeaways
Quartz tubes contain over 99% pure silica, making them highly resistant to heat and chemicals. This purity ensures better performance in demanding environments.
Glass tubes have lower temperature resistance and can soften at temperatures below 820°C. Quartz tubes can withstand temperatures up to 1200°C, making them ideal for high-temperature applications.
Quartz tubes have significantly lower impurity levels, which minimizes contamination risks in sensitive processes like semiconductor manufacturing and chemical processing.
The service life of quartz tubes at high temperatures ranges from 18 to 36 months, while glass tubes last only 3 to 8 months. This longevity reduces replacement costs and downtime.
Choosing the right tube material depends on application needs. Quartz is best for extreme conditions, while glass is suitable for moderate environments and decorative uses.
How Does Compositional Difference Between Quartz and Glass Tubes Drive All Performance Variations?

The difference between glass tube and quartz tube starts with their basic material makeup. This compositional gap leads to major changes in how each tube performs in real-world settings. Understanding these differences helps users choose the right tube for their needs.
Pure Silica Network in Quartz (99.98% SiO₂) Versus Mixed-Oxide Structure in Glass
Quartz tubes, such as those from TOQUARTZ, contain over 99.98% pure silicon dioxide. This high purity gives quartz tubes unique properties, including high thermal stability and strong chemical resistance. Glass tubes, in contrast, use a mixed-oxide structure with much lower silica content.
Quartz tubes: Nearly 100% SiO₂
Glass tubes: 73% SiO₂ (soda-lime), rest are additives
High silica content = higher melting point and better durability
The difference between glass tube and quartz tube becomes clear when comparing their silica content. Fused quartz tubes can handle higher temperatures and resist harsh chemicals better than glass tubes. This makes quartz the preferred choice for demanding laboratory and industrial applications.
Network Modifier Ions (Na⁺, Ca²⁺) Creating Performance Limitations in Glass
Glass tubes include network modifier ions like sodium (Na⁺) and calcium (Ca²⁺), which break up the silica network. These ions lower the melting point and make glass easier to shape, but they also reduce chemical and thermal resistance. Quartz tubes do not contain these modifiers, so their structure stays strong under stress.
Property | Quartz Tubes | Glass Tubes |
|---|---|---|
Network Modifiers | None | Na⁺, Ca²⁺ present |
Melting Point (°C) | ~1730 | 696–820 |
Chemical Resistance | Excellent | Moderate |
The presence of network modifiers explains why glass tubes cannot match the performance of quartz tubes in extreme environments. This structural difference is a key factor in the difference between glass tube and quartz tube.
Impurity Content Impact: <20 ppm Total (Quartz) Versus 500-3000 ppm Fe₂O₃ (Glass)
Impurity levels also play a major role in tube performance. Quartz tubes have less than 20 parts per million (ppm) total impurities, while glass tubes may contain 500–3000 ppm of iron oxide (Fe₂O₃) and other contaminants. These impurities can affect optical clarity and chemical stability.
Quartz tubes: Ultra-low impurity content
Glass tubes: Higher levels of iron and other metals
Low impurities = better optical and chemical performance
Low impurity content in quartz tubes ensures reliable results in sensitive applications, such as optics and semiconductor manufacturing. Glass tubes, with higher impurity levels, may not provide the same level of purity or performance.
Why Do Temperature Capabilities Differ by 430-700°C Between Quartz and Glass Tubes?

Temperature resistance stands as one of the most important differences between quartz and glass tubes. The ability to withstand heat directly affects where each material can be used safely and effectively. Understanding these differences helps users select the right tube for high-temperature or thermal cycling environments.
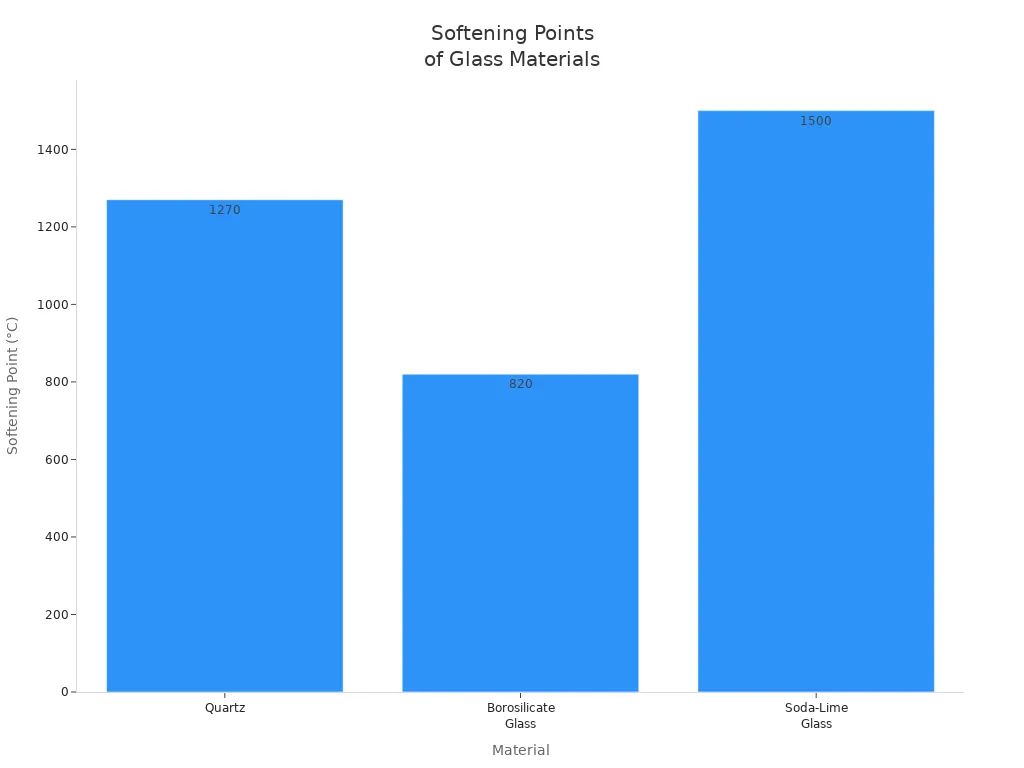
Softening Point Differences: 1730°C (Quartz) vs 820°C (Borosilicate) vs 696°C (Soda-Lime)
The softening point marks the temperature where a tube begins to lose its shape. Quartz tubes, such as those from TOQUARTZ, have a softening point of about 1730°C, while borosilicate glass softens at 820°C and soda-lime glass at 696°C. This large gap explains why quartz tubes perform better in extreme heat.
Material | Softening Point (°C) | Maximum Use Temperature (°C) | Melting Point (°C) |
|---|---|---|---|
Quartz | 1270 | 1200 (3 hours) | 1670 |
Borosilicate Glass | 820 | 520 | 1260 |
Soda-Lime Glass | 696 | 500 | 1500-1700 |
Quartz’s high softening point allows it to maintain its structure and clarity even during long exposure to high temperatures.
Key summary:
Quartz tubes withstand much higher temperatures than glass tubes.
Borosilicate and soda-lime glass tubes deform at lower temperatures.
Thermal Expansion Impact: 0.5 vs 3.3 vs 9.0 × 10⁻⁶ K⁻¹ on Thermal Cycling
Thermal expansion measures how much a material grows when heated. Quartz tubes have a very low coefficient of thermal expansion (about 0.55 × 10⁻⁶/K), while borosilicate glass is higher at 3.25 × 10⁻⁶/K, and soda-lime glass is even higher. This means quartz tubes resist cracking and breaking during rapid temperature changes.
Quartz tubes: Minimal expansion, excellent thermal shock resistance
Borosilicate glass: Moderate expansion, good for some lab uses
Soda-lime glass: High expansion, poor thermal shock resistance
A low expansion rate makes quartz tubes ideal for repeated heating and cooling cycles, such as in furnaces or semiconductor processing.
Summary:
Low thermal expansion in quartz prevents failure during thermal cycling.
Service Life at High Temperature: 18-36 Months vs 3-8 Months vs Immediate Failure
Service life at high temperature shows how long a tube can last before it fails. Quartz tubes can operate for 18 to 36 months at high temperatures, while borosilicate glass tubes last only 3 to 8 months, and soda-lime glass may fail immediately under the same conditions. This difference between glass tube and quartz tube becomes critical in industrial and laboratory settings.
Material | Typical Service Life at High Temp |
|---|---|
Quartz | 18–36 months |
Borosilicate Glass | 3–8 months |
Soda-Lime Glass | Immediate failure |
Longer service life reduces replacement costs and downtime for users.
Key summary:
Quartz tubes offer much longer service life at high temperatures.
How Does Chemical Resistance Create 50-1500× Performance Gap in Acid Processing?
Chemical resistance plays a critical role in determining the suitability of tubes for acid processing. Quartz tubes and glass tubes show dramatic differences in how they respond to corrosive chemicals, especially acids. This section explains why quartz tubes outperform glass tubes by such a large margin in harsh chemical environments.
Alkali Leaching Mechanism: 4-18% Na₂O/CaO in Glass vs <0.01% in Quartz
Glass tubes contain significant amounts of sodium oxide (Na₂O) and calcium oxide (CaO), which can leach into solutions during acid exposure. These network modifiers weaken the glass structure and allow alkali ions to escape, leading to contamination and faster degradation. Quartz tubes, with less than 0.01% alkali content, remain stable and do not release harmful ions.
Quartz tubes maintain purity in acid environments
Glass tubes release sodium and calcium, causing contamination
Low alkali content in quartz prevents leaching
This difference between glass tube and quartz tube becomes especially important in applications where even trace contamination can affect results.
Penetration Rates in Concentrated Acids: 0.02-0.05 mm/year vs 0.8-20 mm/year
Acid penetration rates measure how quickly acids can attack and wear down the tube material. Quartz tubes show extremely low penetration rates, typically 0.02 to 0.05 mm per year, even in strong acids. In contrast, glass tubes can experience rates from 0.8 up to 20 mm per year, resulting in rapid thinning and failure.
Tube Type | Acid Penetration Rate (mm/year) | Durability in Acid |
|---|---|---|
Quartz | 0.02–0.05 | Very High |
Glass | 0.8–20 | Low to Moderate |
Quartz tubes last much longer in acid processing, making them the preferred choice for reactor liners and sample holders in chemical plants.
Contamination Impact: <1 ppb (Quartz) vs 10-50 ppb Na/B Leaching (Glass)
Contamination from leached elements can compromise sensitive experiments and industrial processes. Research shows that glass tubes can leach 10 to 50 parts per billion (ppb) of sodium and boron into solutions, while quartz tubes keep contamination below 1 ppb. This ultra-low impurity level ensures reliable results in semiconductor manufacturing and analytical laboratories.
Quartz tubes minimize contamination risk
Glass tubes introduce measurable sodium and boron
High purity in quartz supports critical applications
Quartz tubes are essential in environments where purity and chemical resistance directly impact product quality and safety.
What Material Science Principles Enable Superior Performance in Pure Quartz Versus Additive-Containing Glass?
Material science explains why pure quartz tubes outperform glass tubes in demanding environments. The atomic structure, presence of additives, and uniformity of composition all play key roles. Understanding these principles helps users make informed choices for high-performance applications.
Covalent Network Structure Providing Maximum Bond Strength in Pure Silica
Pure quartz tubes feature a continuous covalent network of silicon and oxygen atoms. This structure creates strong Si–O bonds, which give quartz its high melting point and resistance to mechanical stress. The absence of weak points in the network allows quartz tubes to maintain their integrity under extreme conditions.
The following table highlights the superior properties of fused silica compared to additive-containing glass:
Property | Description |
|---|---|
Composition | |
Melting Point | Very high, more refractory than glass |
Flexural Strength | 54.8 MPa, indicating high durability |
Fracture Toughness | 1 MPa·m¹/², resists cracking |
This robust network structure explains why quartz tubes, such as those from TOQUARTZ, deliver reliable performance in high-stress environments.
Network Modifier Effects Creating Thermal and Chemical Vulnerabilities
Glass tubes contain network modifiers like sodium and calcium oxides. These additives disrupt the Si–O–Si linkages, making the glass structure less connected and more vulnerable to heat and chemicals. As a result, glass tubes show lower viscosity, reduced melting points, and limited UV transmission.
Network modifiers also increase the risk of thermal shock and chemical attack. The disrupted network allows ions to leach out, which can lead to contamination and faster degradation in harsh environments. This makes glass tubes less suitable for applications that demand high purity and stability.
Additives weaken the glass structure
Lower resistance to heat and chemicals
Increased risk of contamination and failure
These vulnerabilities highlight the importance of choosing pure quartz tubes for critical processes.
Compositional Homogeneity Enabling Predictable Long-Term Performance
Quartz tubes offer exceptional compositional homogeneity, which ensures consistent performance over time. This uniformity allows quartz tubes to withstand continuous operation at temperatures up to 1200°C and resist thermal shock exceeding 1000°C temperature differentials. Glass tubes, with their variable composition, soften at lower temperatures and fail under rapid temperature changes.
The table below compares the long-term performance of quartz and glass tubes:
Property | Quartz Tubes | Glass Tubes |
|---|---|---|
Continuous Operation Temp | Up to 1200°C | Softens at 600°C |
Thermal Shock Resistance | >1000°C differential | Fails above 300°C |
UV Transmission | Superior | Limited |
Chemical Inertness | High | Lower |
This predictable performance makes quartz tubes the preferred choice for semiconductor, aerospace, and laser applications.
Cost and Availability Note:
Quartz tubes, including TOQUARTZ fused quartz, generally cost more than standard glass tubes due to advanced manufacturing and higher purity. TOQUARTZ offers both standard sizes and custom solutions, ensuring users can find the right fit for any project.
Which Material Should You Specify Based on Application Temperature, Purity, and Optical Requirements?
Selecting the right tube material depends on the demands of your application. Temperature, purity, and optical requirements all play a role in determining whether quartz or glass is the best fit. Understanding these factors helps engineers and scientists make informed decisions for reliable performance.
High-Temperature Applications (>700°C) Requiring Quartz: Furnaces, Semiconductor, CVD
Quartz tubes excel in environments where temperatures exceed 700°C. Semiconductor manufacturing, chemical vapor deposition (CVD), and diffusion furnaces rely on quartz for its stability and purity. Data shows that quartz tubes maintain integrity at temperatures up to 1200°C, while glass tubes soften and fail above 500°C.
Application Area | Role of Quartz Tubes |
|---|---|
CVD | Thermal shields for wafer deposition |
Diffusion Furnace | Stable thermal gradients for doping |
Etching Chamber | Protection against corrosive gases |
Quartz tubes support high throughput and reduce downtime in these industries. Their longevity and resistance to degradation make them essential for advanced systems.
UV Optical Applications (<320 nm) Mandating Quartz: Sterilization, Photochemistry, Lasers
Quartz tubes provide superior UV transmission below 320 nm, which is critical for sterilization and photochemistry. Standard glass tubes block much of this UV light, limiting their use in applications like germicidal lamps and laser optics. Engineers choose quartz for its ability to transmit specific UV wavelengths needed for precise scientific work.
High UV transmittance for sterilization
Essential for photochemical reactions
Preferred in laser and semiconductor photolithography
Quartz tubes enable accurate control of UV exposure, supporting advanced research and industrial processes.
Chemical Processing (>50% Acid) Demanding Quartz: Wet Cleaning, Digestion Systems
Chemical processing with strong acids requires tubes that resist corrosion and contamination. Quartz tubes contain less than 0.01% alkali, preventing leaching and ensuring purity. Glass tubes, with higher alkali content, can release sodium and calcium, leading to contamination and rapid wear.
Tube Type | Acid Penetration Rate (mm/year) | Contamination Risk |
|---|---|---|
Quartz | 0.02–0.05 | Very Low |
Glass | 0.8–20 | Moderate to High |
Quartz tubes last longer and maintain sample integrity in wet cleaning and digestion systems. Their chemical durability supports critical laboratory and industrial processes.
Cost-Optimized Applications Where Glass Adequate: Lab Glassware, Visible Optics, Moderate Conditions
Glass tubes offer a cost-effective solution for applications with moderate temperature and purity requirements. Everyday laboratory glassware, decorative items, and visible optics often use glass due to its affordability and ease of shaping. These applications do not demand the extreme properties of quartz.
Suitable for household and decorative uses
Adequate for moderate lab conditions
Lower cost for non-critical environments
Glass tubes provide reliable performance where high temperature or chemical resistance is not required.
Total Cost of Ownership Framework: When Higher Unit Cost Delivers Lower Lifecycle Expense
Choosing quartz tubes may involve a higher initial investment, but their durability and long service life reduce replacement costs. Data shows that quartz tubes can operate for 18–36 months in high-temperature settings, while glass tubes may need frequent replacement. This difference lowers overall expenses and minimizes downtime.
Property | Quartz Tubes | Glass Tubes |
|---|---|---|
Initial Cost | Higher | Lower |
Service Life | 18–36 months | 3–8 months |
Replacement Rate | Low | High |
Quartz tubes deliver long-term value in demanding environments, making them a smart choice for critical applications.
Choosing between glass tubes and quartz tubes depends on the application’s demands. Quartz tubes offer higher temperature tolerance, purity, and optical clarity, making them ideal for challenging environments. Glass tubes suit general or decorative uses where extreme conditions are not present. The table below highlights the main factors:
Factor | Quartz Tubes | Glass Tubes |
|---|---|---|
Temperature | Extreme resistance | Limited range |
Purity | High | Lower |
Optical Qualities | Excellent | Moderate |
Users should match tube selection to temperature, purity, and optical needs for the best results. The difference between glass tube and quartz tube can impact safety, performance, and cost.
FAQ
What is the main difference between quartz tubes and glass tubes?
Quartz tubes contain over 99% pure silica. Glass tubes use a mix of silica and other compounds. This difference affects temperature resistance, chemical durability, and purity.
Can both quartz and glass tubes handle high temperatures?
Quartz tubes withstand temperatures up to 1200°C. Glass tubes soften below 820°C. Quartz performs better in furnaces and semiconductor processes.
Which tube type offers better chemical resistance?
Quartz tubes resist acids and alkalis much better than glass tubes. Data shows quartz tubes have acid penetration rates as low as 0.02 mm/year, while glass tubes reach up to 20 mm/year.
Are quartz tubes more expensive than glass tubes?
Quartz tubes cost more due to their purity and advanced manufacturing. Glass tubes offer a lower price for general applications.
When should someone choose glass tubes instead of quartz tubes?
Glass tubes work well for moderate temperatures, visible optics, and decorative uses. Quartz tubes suit high-temperature, high-purity, or UV applications.





