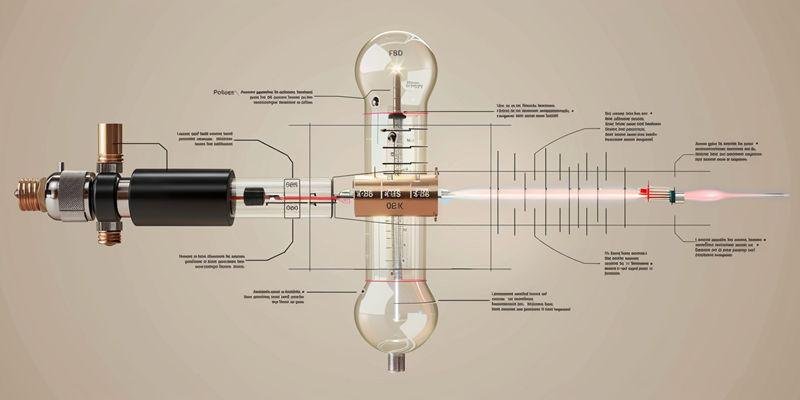
وتلعب أنابيب الكوارتز دورًا حاسمًا في تجربة التأثير الكهروضوئي لأنها تسمح للأشعة فوق البنفسجية بالوصول إلى السطح المعدني، وهو ما يحجبه الزجاج العادي. تضمن هذه الخاصية الفريدة للكوارتز أن يوفر إعداد التأثير الكهروضوئي لأنبوب الكوارتز نتائج دقيقة وموثوقة في كل مرة. ويستفيد الباحثون أيضًا من بساطة الكوارتز وثباته، مما يدعم القياسات المتسقة على مدى فترات طويلة.
الوجبات الرئيسية
يسمح زجاج الكوارتز بمرور الضوء فوق البنفسجي، وهو أمر ضروري لتجربة التأثير الكهروضوئي. يحجب الزجاج العادي هذا الضوء، مما يمنع الحصول على نتائج دقيقة.
لا يحدث التأثير الكهروضوئي إلا عندما يكون للضوء طاقة كافية، وهو ما يعتمد على طوله الموجي. الضوء فوق البنفسجي ضروري لتحرير الإلكترونات من المعادن.
استخدام أنابيب مفرغة من الهواء مع نوافذ كوارتز يمنع تداخل الهواء، مما يسمح للإلكترونات بالانتقال بحرية ويضمن قياسات دقيقة.
يقاوم زجاج الكوارتز التغيرات الكيميائية وامتصاص الرطوبة، مما يحافظ على انتقال الأشعة فوق البنفسجية بثبات بمرور الوقت لإجراء تجارب موثوقة على المدى الطويل.
اختيار درجة الكوارتز المناسبة أمر بالغ الأهمية. النوع الثالث من الكوارتز هو الأفضل لتجارب الأشعة فوق البنفسجية العميقة، بينما النوع الأول من الكوارتز مناسب للأطوال الموجية القياسية للأشعة فوق البنفسجية.
ما هو التأثير الكهروضوئي ولماذا يحتاج إلى أطوال موجية ضوئية محددة؟
إن التأثير الكهروضوئي كيف يمكن للضوء أن يتسبب في خروج الإلكترونات من سطح فلز. ولا تحدث هذه العملية إلا عندما يكون للضوء طاقة كافية، وهو ما يعتمد على طوله الموجي. توضِّح تجربة التأثير الكهروضوئي لأنبوب الكوارتز هذا المبدأ باستخدام الضوء فوق البنفسجي الذي لا يستطيع الزجاج العادي نقله.
نظرية الفوتون لأينشتاين ومتطلبات تردد العتبة
شرح أينشتاين التأثير الكهروضوئي باقتراح أن الضوء يتكون من جسيمات تسمى الفوتونات. ويحمل كل فوتون كمية محددة من الطاقة، والفوتونات التي تزيد طاقتها عن عتبة معينة هي فقط التي يمكنها تحرير الإلكترونات من المعدن. وتعتمد هذه العتبة على تردد الضوء وليس على شدته.
لاحظ العلماء عدة نتائج رئيسية التي تدعم نظرية أينشتاين. على سبيل المثال، تنقذف الإلكترونات على الفور عندما يكون تردد الضوء مرتفعًا بما فيه الكفاية، وزيادة شدة الضوء تزيد فقط من عدد الإلكترونات وليس من طاقتها. وتعتمد الطاقة الحركية للإلكترونات المقذوفة على تردد الضوء، مما يدل على أن انتقال الطاقة مكمّم.
الملاحظة | الوصف |
|---|---|
تردد العتبة | لا تُقذف أي إلكترونات أقل من تردد معين، بغض النظر عن شدتها. |
الطرد اللحظي | تظهر الإلكترونات على الفور عند استيفاء العتبة. |
التناسب مع الكثافة | ينتج الضوء الأكثر كثافة المزيد من الإلكترونات، وليس طاقة أعلى. |
استقلالية الطاقة الحركية | تعتمد طاقة الإلكترون على التردد وليس الشدة. |
معادلة الطاقة | KE = hf - BE يوضِّح العلاقة بين طاقة الفوتون وانبعاث الإلكترونات. |
تفسر هذه النتائج لماذا يتطلب إعداد التأثير الكهروضوئي لأنبوب الكوارتز تحكمًا دقيقًا في الطول الموجي للضوء.
مواد الكاثود الضوئي الشائعة وقيم دالة عملها
تحتاج المعادن المختلفة إلى كميات مختلفة من الطاقة لتحرير الإلكترونات، وهي خاصية تسمى دالة الشغل. تحدد دالة الشغل الحد الأدنى لطاقة الفوتون اللازمة لحدوث التأثير الكهروضوئي. فلزات مثل السيزيوم والبوتاسيوم والصوديوم لها دوال شغل منخفضة، مما يجعلها مثالية للتجارب.
يؤثر اختيار المعدن على مصدر الضوء الذي يعمل بشكل أفضل. على سبيل المثال، يحتاج الصوديوم والبوتاسيوم إلى طاقة أقل من الزنك أو البلاتين، لذلك من الضروري استخدام ضوء فوق بنفسجي بطول موجي مناسب. وغالبًا ما تستخدم تجربة التأثير الكهروضوئي لأنبوب الكوارتز هذه المعادن لأن وظائف عملها تتطابق مع طاقة فوتونات الأشعة فوق البنفسجية.
العنصر | دالة العمل (Φ) (فولت) |
|---|---|
2.36 | |
البوتاسيوم (K) | 2.3 |
السيزيوم (Cs) | 1.95 |
يختار الباحثون المعدن بناءً على مصدر الضوء المتاح والنتائج التجريبية المطلوبة.
لماذا تعتبر الأشعة فوق البنفسجية ضرورية لانبعاث الإلكترونات الضوئية
الأشعة فوق البنفسجية حاسمة في التأثير الكهروضوئي لأن لديها طاقة كافية للتغلب على دالة الشغل لمعظم المعادن. الأطوال الموجية الأقصر تعني طاقة فوتون أعلى، وهو أمر ضروري لتحرير الإلكترونات من سطح المعدن. لا يمتلك الضوء المرئي عادةً طاقة كافية، لذلك لا يمكنه إحداث التأثير في معظم الحالات.
توفر الأشعة فوق البنفسجية الطاقة اللازمة لانبعاث الإلكترونات.
تتوافق الأطوال الموجية الأقصر مع طاقة فوتون أعلى.
غالبًا ما يفشل الضوء المرئي في إحداث التأثير في المعادن الشائعة.
نظرًا لأن الأشعة فوق البنفسجية هي الوحيدة القادرة على توفير الطاقة اللازمة، تعتمد تجربة التأثير الكهروضوئي لأنبوب الكوارتز على الكوارتز لنقل هذه الأطوال الموجية. وهذا يضمن نتائج دقيقة وموثوقة في كل مرة.
لماذا ينقل زجاج الكوارتز الأشعة فوق البنفسجية بينما يحجبها الزجاج العادي؟

يبدو زجاج الكوارتز والزجاج العادي متشابهين، لكن قدرتهما على نقل الأشعة فوق البنفسجية (UV) مختلفة تمامًا. ويأتي هذا الاختلاف من التركيب الكيميائي الفريد والبنية الفريدة لكل مادة. يساعد فهم السبب الذي يجعل الكوارتز يسمح بمرور الأشعة فوق البنفسجية بينما يحجبها الزجاج العادي في تفسير نجاح أنبوب الكوارتز تجربة التأثير الكهروضوئي.
امتصاص البنية الإلكترونية في شوائب أكسيد الفلزات
يحتوي الزجاج العادي على شوائب أكسيد المعادن التي تمتص الأشعة فوق البنفسجية. وتدخل هذه الشوائب، مثل أكاسيد الحديد والصوديوم والكالسيوم، نطاقات طاقة خاصة في بنية الزجاج. عندما يصطدم ضوء الأشعة فوق البنفسجية بالزجاج العادي، تمتص الإلكترونات الموجودة في هذه الأكاسيد المعدنية الطاقة، مما يجعل الزجاج يحجب الأطوال الموجية للأشعة فوق البنفسجية.
يحدث الامتصاص لأن البنية الإلكترونية لهذه الشوائب تخلق نطاقات امتصاص عند أطوال موجية محددة للأشعة فوق البنفسجية. على سبيل المثال، تحتوي أيونات الحديد (Fe²⁺ و Fe³⁺) في الزجاج على نطاقات انتقال الشحنة التي تمتص الأشعة فوق البنفسجية بقوة. وتعني هذه العملية، التي تسمى الأكسدة الضوئية، أن معظم فوتونات الأشعة فوق البنفسجية لا تمر أبدًا عبر الزجاج العادي، مما يجعله غير مناسب للتجارب التي تحتاج إلى انتقال الأشعة فوق البنفسجية.
يوضح ملخص هذه العملية سبب حجب الزجاج العادي للأشعة فوق البنفسجية:
تخلق شوائب أكسيد الفلز نطاقات امتصاص في نطاق الأشعة فوق البنفسجية.
تمتص أيونات الحديد فوتونات الأشعة فوق البنفسجية من خلال آليات نقل الشحنة.
تعمل الأكسدة الضوئية على تحويل طاقة الأشعة فوق البنفسجية إلى حرارة، مما يحول دون انتقالها.
وبسبب هذه التأثيرات، يمكن أن تمر كمية صغيرة فقط من ضوء الأشعة فوق البنفسجية عبر الزجاج العادي، مما يمنع حدوث التأثير الكهروضوئي في هذه التركيبات.
طاقة فجوة الحزمة وانتقال الفوتون بالأشعة فوق البنفسجية في SiO₂ النقي
إن زجاج الكوارتز النقي، المصنوع بالكامل تقريبًا من ثاني أكسيد السيليكون (SiO₂)، له بنية إلكترونية مختلفة تمامًا. فطاقة فجوة الحزمة في SiO₂ SiO₂ أعلى بكثير من طاقة فوتونات الأشعة فوق البنفسجية المستخدمة في التجارب الكهروضوئية. وتعني فجوة الحزمة الكبيرة هذه أن الأشعة فوق البنفسجية لا تحتوي على طاقة كافية لإثارة الإلكترونات في الكوارتز، وبالتالي يمر الضوء بسهولة.
إن حافة امتصاص SiO₂ النقي تقع في عمق طيف الأشعة فوق البنفسجية. نظرًا لأن فجوة النطاق واسعة جدًا، لا يمكن امتصاص سوى الفوتونات ذات الطاقة العالية للغاية - أعلى بكثير من تلك المستخدمة في التجارب النموذجية. ونتيجة لذلك، يظل زجاج الكوارتز شفافًا للأشعة فوق البنفسجية في النطاق اللازم للتأثير الكهروضوئي لأنبوب الكوارتز.
يقارن الجدول أدناه بين أداء الإرسال بالأشعة فوق البنفسجية للزجاج العادي وزجاج الكوارتز، ويوضح كيف تؤدي طاقة فجوة النطاق إلى نتائج مختلفة:
نوع الزجاج | أداء انتقال الأشعة فوق البنفسجية |
|---|---|
زجاج عادي | انتقال أقل للأشعة فوق البنفسجية، أقل من 80% في الأشعة فوق البنفسجية |
زجاج الكوارتز | انتقال فائق للأشعة فوق البنفسجية، أكثر من 80% في الأشعة فوق البنفسجية |
يفسر هذا الاختلاف في البنية الإلكترونية سبب كون الكوارتز المادة المفضلة لنقل الأشعة فوق البنفسجية في التجارب العلمية.
مقارنة الانتقال الكمي عند أطوال موجات الأشعة فوق البنفسجية الحرجة
يقيس العلماء مقدار الأشعة فوق البنفسجية التي تمر عبر أنواع مختلفة من الزجاج عند أطوال موجية مهمة. وينقل زجاج الكوارتز أكثر من 801 تيرابايت 3 تيرابايت من الأشعة فوق البنفسجية عند 254 نانومتر و365 نانومتر، وهي أطوال موجية شائعة في تجارب التأثير الكهروضوئي. من ناحية أخرى، يحجب الزجاج العادي كل ضوء الأشعة فوق البنفسجية تقريبًا تحت 300 نانومتر ويفقد نصف انتقاله عند 350 نانومتر.
تُظهر البيانات المختبرية أن كوفيت الكوارتز يسمح بمرور الضوء من 190 نانومتر حتى 2500 نانومتر، مما يجعلها مثالية لتجارب الأشعة فوق البنفسجية. يعمل الزجاج العادي بشكل جيد فقط في النطاق المرئي والأشعة تحت الحمراء القريبة، بدءًا من حوالي 320 نانومتر. وهذا يعني أن تجربة التأثير الكهروضوئي لأنبوب الكوارتز لا يمكن أن تنجح إلا عند استخدام الكوارتز، لأن الزجاج العادي يحجب ضوء الأشعة فوق البنفسجية الضروري.
ينقل الكوارتز أكثر من 80% من الأشعة فوق البنفسجية عند 254 نانومتر و365 نانومتر.
يحجب الزجاج العادي كل الأشعة فوق البنفسجية التي تقل عن 300 نانومتر تقريبًا.
تتطلب تجارب التأثير الكهروضوئي انتقالًا عاليًا للأشعة فوق البنفسجية للحصول على نتائج دقيقة.
تسلط هذه الحقائق الضوء على أهمية اختيار زجاج الكوارتز للتجارب التي تعتمد على انتقال الأشعة فوق البنفسجية.
لماذا يتطلب التأثير الكهروضوئي أنابيب مفرغة ذات نوافذ كوارتز؟

تتطلب تجارب التأثير الكهروضوئي بيئة مضبوطة لضمان الحصول على نتائج دقيقة. يستخدم العلماء أنابيب مفرغة مع نوافذ كوارتز لمنع التداخل من الهواء والحفاظ على ظروف مستقرة لحركة الإلكترونات. يتيح الجمع بين التفريغ والكوارتز قياسًا دقيقًا وموثوقية طويلة الأجل في إعداد التأثير الكهروضوئي لأنبوب الكوارتز.
فيزياء تصادم جزيء الإلكترون والغاز ومتوسط المسار الحر
يمكن أن تتصادم الإلكترونات المنطلقة من سطح المعدن مع جزيئات الغاز إذا بقي الهواء داخل الأنبوب. تقلل هذه التصادمات من عدد الإلكترونات التي تصل إلى الكاشف وتشوه القياس. وتؤدي إزالة الهواء من الأنبوب إلى زيادة متوسط المسار الحر، مما يسمح للإلكترونات بالانتقال مباشرة إلى المجمع دون تداخل.
عند وجود الهواء، تفقد الإلكترونات الطاقة من خلال التصادمات غير المرنة مع جزيئات الغاز. هذا الفقدان للطاقة يجعل من الصعب قياس الطاقة الحركية الحقيقية للإلكترونات الضوئية، وهو أمر ضروري للتحقق من معادلة أينشتاين. وقد وجد العلماء أنه عند الضغط الجوي، يبلغ متوسط المسار الحر للإلكترونات حوالي 68 نانومترًا فقط، في حين أن المسافة بين المهبط والمصعد أكبر بكثير.
يبرز جدول موجز تأثير الهواء على سفر الإلكترونيات:
الحالة | متوسط المسار الحر | كشف الإلكترون الضوئي | السببية |
|---|---|---|---|
أنبوب مملوء بالهواء | 68 نانومتر | منخفضة جداً | تعمل التصادمات على تشتيت الإلكترونات، مما يقلل من الإشارة |
أنبوب الإخلاء | > 100 متر | عالية | تنتقل الإلكترونات بحرية، قياس دقيق ودقيق |
يوضح هذا الجدول لماذا يستخدم العلماء دائمًا الأنابيب المفرغة لإجراء تجارب التأثير الكهروضوئي الموثوق بها.
متطلبات الفراغ لانتقال الإلكترونات الضوئية دون عوائق
ويضمن التفريغ عالي الجودة انتقال الإلكترونات الضوئية من السطح المعدني إلى المجمع دون فقدان الطاقة. يزيل الفراغ جميع جزيئات الغاز تقريبًا، وبالتالي يمكن للإلكترونات الانتقال دون عوائق عبر الأنبوب. يسمح هذا الإعداد للباحثين بقياس الطاقة الحركية الحقيقية وإمكانات التوقف للإلكترونات المنبعثة.
وتعتمد القياسات الدقيقة على الحفاظ على فراغ عند أو أقل من 10 ⁵ تور، مما يزيد من متوسط المسار الحر للإلكترونات إلى أكثر من 100 متر. وتتجاوز هذه المسافة بكثير حجم الأنبوب التجريبي، لذلك تصل جميع الإلكترونات الضوئية تقريبًا إلى الكاشف دون تشتت. ويعتمد العلماء على هذا الشرط للتحقق من العلاقة بين طاقة الفوتون وانبعاث الإلكترونات.
يزيل الفراغ التصادمات بين الإلكترونات والغازات
يضمن طول متوسط المسار الحر المتوسط الطويل اكتشافًا دقيقًا
تفريغ مستقر يدعم نتائج متسقة
نظرًا لأهمية التفريغ، يتحقق الباحثون دائمًا من الضغط داخل الأنبوب قبل بدء تجربة التأثير الكهروضوئي لأنبوب الكوارتز.
لماذا تمكن الخصائص الحرارية للكوارتز من تصنيع الأنابيب المفرغة من الهواء
يتميز زجاج الكوارتز بخصائص حرارية فريدة من نوعها تجعله مثاليًا لتصنيع أنابيب التفريغ. حيث تزداد الموصلية الحرارية مع ارتفاع درجة الحرارة، مما يساعد على التحكم في الحرارة أثناء عملية الختم. كما تتحمل المادة أيضًا درجات الحرارة العالية دون أن تتشقق، مما يضمن ختم تفريغ قوي وطويل الأمد.
أثناء عملية التصنيع، يقوم الفنيون بتسخين الكوارتز إلى درجات حرارة أعلى من 1200 درجة مئوية لإنشاء أختام محكمة الإغلاق. ترتفع الموصلية الحرارية للكوارتز من حوالي 1.35 جول/(م-ث-س-ج) في درجة حرارة الغرفة إلى 1.82 جول/(م-ث-س-ج) عند 450 درجة مئوية، وهو ما يتوافق مع احتياجات إنتاج الأنابيب المفرغة من الهواء. وتمنع هذه الخاصية حدوث صدمة حرارية وتسمح للأنبوب بالحفاظ على تفريغه على مدى سنوات عديدة.
يقاوم الكوارتز التشقق أثناء الإغلاق في درجات الحرارة العالية
تدعم الموصلية الحرارية التوزيع المتساوي للحرارة
تحافظ الأختام القوية على سلامة التفريغ للاستخدام طويل الأمد
تفسر هذه الميزات سبب كون الكوارتز المادة المفضلة لبناء الأنابيب المفرغة في تجارب التأثير الكهروضوئي.
لماذا يُعد الاستقرار الكيميائي للكوارتز مهمًا للقياسات الكهروضوئية طويلة المدى؟
يبرز زجاج الكوارتز في تجارب التأثير الكهروضوئي لأنه يقاوم التغيرات الكيميائية التي يمكن أن تؤثر على النتائج بمرور الوقت. في المقابل، يتفاعل الزجاج العادي مع الرطوبة والمواد الكيميائية، مما قد يقلل من انتقال الأشعة فوق البنفسجية ويغير السطح المعدني. تعتمد القياسات الموثوقة طويلة الأجل على الثبات الذي يوفره الكوارتز.
آليات النض القلوي السطحي في الزجاج العادي
الرشح القلوي يضعف الأسطح الزجاجية العادية أثناء التجارب الكهروضوئية. وتبدأ العملية بالتبادل الأيوني، حيث تتبادل أيونات الفلزات القلوية أماكنها مع أيونات الهيدروجين، مما يرفع قلوية المحلول. ومع ارتفاع الأس الهيدروجيني عن 9، تتفكك شبكة السيليكا، مكونة أيونات Si(OH)6²- المذابة، وتتعرض الطبقة المرشحة للتوتر بسبب صغر حجم أيونات الهيدروجين، مما قد يسبب التشقق والمزيد من الرشح.
يؤدي هذا التغير الكيميائي إلى تكوين طبقة سطحية هشة. وتسمح التشققات وزيادة خشونة السطح بتغلغل المزيد من الرطوبة والملوثات، مما يسرّع من عملية التدهور. وبمرور الوقت، تقلل هذه التغييرات من دقة وموثوقية القياسات الكهروضوئية.
يبدأ النض القلوي بالتبادل الأيوني
يتبع التوتر السطحي والتشقق
يسمح التدهور يسمح بمزيد من التلوث
تفسر هذه التأثيرات سبب عدم قدرة الزجاج العادي على الحفاظ على أداء مستقر في التجارب طويلة المدى.
كيف يقلل امتصاص الرطوبة من انتقال الأشعة فوق البنفسجية بمرور الوقت
يقلل امتصاص الرطوبة على الأسطح الزجاجية من انتقال الأشعة فوق البنفسجية ويؤثر على التأثير الكهروضوئي لأنبوب الكوارتز. تشكل جزيئات الماء أغشية رقيقة على الزجاج، والتي تشتت ضوء الأشعة فوق البنفسجية وتمتصه، مما يقلل من الكمية التي تصل إلى السطح المعدني. تصبح هذه العملية أكثر حدة مع تقادم الزجاج أو إذا كانت البيئة رطبة.
لاحظ الباحثون أن انتقال الأشعة فوق البنفسجية عند 254 نانومتر يمكن أن ينخفض بمقدار 15-401 تيرابايت في غضون عام عند تعريض الزجاج العادي لهواء المختبر. يسبب هذا الفقدان في الإرسال أخطاء منهجية في قياس إمكانات التوقف والتيارات الضوئية، مما يجعل من الصعب التحقق من معادلة أينشتاين بدقة. ويكون هذا التأثير ملحوظاً بشكل خاص في التجارب التي تتطلب توصيل ضوء الأشعة فوق البنفسجية بدقة وثبات.
العامل | التأثير على انتقال الأشعة فوق البنفسجية | السببية |
|---|---|---|
امتصاص الرطوبة | ينخفض بمرور الوقت | تعمل الأغشية المائية على تشتيت الأشعة فوق البنفسجية وامتصاصها |
شيخوخة السطح | تسريع الخسارة | المزيد من الخشونة، والمزيد من احتباس الماء |
ولهذا السبب، يفضل العلماء زجاج الكوارتز، الذي يقاوم امتصاص الرطوبة ويحافظ على انتقال الأشعة فوق البنفسجية العالية.
مقاومة زجاج الكوارتز للهجوم الكيميائي والتلوث السطحي
يقاوم زجاج الكوارتز الهجوم الكيميائي والتلوث السطحي، مما يجعله مثاليًا للقياسات الكهروضوئية طويلة الأمد. تُظهر البيانات التجريبية أنه حتى بعد التعرض بالنسبة لعوامل التنظيف القوية مثل Ce(IV)/HNO₃، تظل أسطح الكوارتز مسطحة وسليمة، دون تآكل مدمر. تضمن هذه المتانة أن يستمر إعداد التأثير الكهروضوئي لأنبوب الكوارتز في تقديم نتائج دقيقة عامًا بعد عام.
يمنع السطح الأملس للكوارتز تراكم الملوثات التي يمكن أن تشتت أو تمتص الأشعة فوق البنفسجية. وعلى عكس الزجاج العادي، لا يحدث للكوارتز تشققات أو خشونة من التعرض للمواد الكيميائية، لذا فهو يحافظ على وضوحه البصري. يمكن للباحثين الاعتماد على الكوارتز لتوفير انتقال ثابت للأشعة فوق البنفسجية وقيم دالة عمل ثابتة لسطح المعدن.
الكوارتز يقاوم التآكل الكيميائي
يظل السطح أملس بعد التنظيف
يدعم الإرسال المستقر للأشعة فوق البنفسجية المستقرة البيانات الموثوقة
هذا الاستقرار الكيميائي هو السبب الرئيسي وراء بقاء الكوارتز المادة المفضلة للتجارب العلمية الصعبة.
كيف يجب على الباحثين اختيار أنابيب الكوارتز لتجارب التأثير الكهروضوئي؟
يجب على الباحثين اختيار أنابيب الكوارتز المناسبة لضمان الحصول على نتائج دقيقة في تجارب التأثير الكهروضوئي. تعتمد عملية الاختيار على الأطوال الموجية للأشعة فوق البنفسجية المستخدمة والمتطلبات المحددة للتجربة. يساعد فهم الاختلافات بين درجات الكوارتز العلماء على مطابقة الأنبوب مع احتياجاتهم.
مطابقة درجة الكوارتز مع متطلبات الطول الموجي التجريبي
ويبدأ اختيار درجة الكوارتز الصحيحة بمعرفة نطاق الطول الموجي للأشعة فوق البنفسجية اللازمة للتجربة. يعمل الكوارتز المنصهر كهربائيًا من النوع الثالث بشكل أفضل لتجارب الأشعة فوق البنفسجية العميقة تحت 220 نانومتر، بينما يناسب الكوارتز المنصهر باللهب من النوع الأول التجارب القياسية التي تستخدم أطوال موجية تتراوح بين 250-400 نانومتر. وتوفر كل درجة مستويات مختلفة من النقاء ومحتوى الهيدروكسيل (OH)، والتي تؤثر على انتقال الأشعة فوق البنفسجية.
يحتوي الكوارتز من النوع الثالث على أقل من 30 جزء في المليون من OH وأكثر من 99.991 جزء في المليون من SiO₂، مما يجعله مثاليًا للتجارب التي تتطلب انتقالًا عاليًا للأشعة فوق البنفسجية بأطوال موجية قصيرة جدًا. يوفر النوع الأول من الكوارتز، الذي يحتوي على 150-200 جزء في المليون من الهيدروكسيل ونقاء أقل قليلاً، انتقالًا ممتازًا لمعظم الأجهزة التعليمية والمعملية. تظهر البيانات المستمدة من الاختبارات المعملية أن الكوارتز من النوع الثالث ينقل أكثر من 901 تيرابايت في المليون من الأشعة فوق البنفسجية عند 200 نانومتر، بينما يحافظ الكوارتز من النوع الأول على انتقال أكثر من 901 تيرابايت في المليون عند 254 نانومتر و365 نانومتر.
يمكن للباحثين استخدام الملخص التالي لتوجيه اختيارهم:
الكوارتز المنصهر كهربائياً من النوع III: أفضل للأشعة فوق البنفسجية العميقة (أقل من 220 نانومتر)، وأعلى درجة نقاء، ومحتوى منخفض من OH.
كوارتز مصهور باللهب من النوع I: مناسبة لـ 250-400 نانومتر، وفعالة من حيث التكلفة، ومعيارية لمعظم المختبرات.
تحقق من انتقال الأشعة فوق البنفسجية: تحقق من بيانات الشركة المصنعة للإرسال >85% عند الطول الموجي المستهدف.
طابق الدرجة على التجربة: اختر بناءً على مصدر الضوء ومادة الكاثود الضوئي.
من خلال اتباع هذه الإرشادات، يضمن العلماء أن تجربة التأثير الكهروضوئي لأنبوب الكوارتز تقدم نتائج موثوقة ودقيقة.
لا تزال أنابيب الكوارتز ضرورية للتأثير الكهروضوئي لأنبوب الكوارتز لأنها توفر انتقالًا لا مثيل له للأشعة فوق البنفسجية وتدعم التفريغ الهوائي وتقاوم التلف الكيميائي. ويضمن الكوارتز وحده بقاء التجارب دقيقة وموثوقة على مدى سنوات عديدة. يختار العلماء الكوارتز لهذه الأسباب:
لا تحتوي السيليكا المنصهرة على أي تلوث معدني تقريبًا، لذا تظل القياسات دقيقة.
يسمح الامتصاص المنخفض بوصول المزيد من الأشعة فوق البنفسجية إلى سطح المعدن.
يحافظ التجانس البصري على ثبات النتائج للدراسات طويلة المدى.
يجب على الباحثين دائماً اختيار الكوارتز لضمان الدقة العلمية.
الأسئلة الشائعة
لماذا لا يمكن استخدام الزجاج العادي في تجارب التأثير الكهروضوئي؟
يحجب الزجاج العادي معظم الأشعة فوق البنفسجية. وهذا يمنع فوتونات الأشعة فوق البنفسجية من الوصول إلى سطح المعدن. وبدون وصول ما يكفي من الأشعة فوق البنفسجية، لا يمكن للإلكترونات أن تتسرب؛ ومن ثم تفشل التجربة.
الأسباب الرئيسية:
امتصاص الأشعة فوق البنفسجية بواسطة الشوائب
إرسال منخفض تحت 350 نانومتر
لا يوجد انبعاث إلكترون ضوئي
كيف يعمل زجاج الكوارتز على تحسين دقة القياس؟
ينقل زجاج الكوارتز أكثر من 85% من الأشعة فوق البنفسجية عند 254 نانومتر و365 نانومتر. يسمح هذا الإرسال العالي بوصول المزيد من الفوتونات إلى المعدن، مما يزيد من التيار الضوئي.
المواد | انتقال الأشعة فوق البنفسجية عند 254 نانومتر |
|---|---|
كوارتز | >85% |
زجاج عادي | <5% |
لماذا يعد التفريغ ضروريًا داخل أنبوب الكوارتز؟
يزيل الفراغ جزيئات الهواء، وبالتالي تنتقل الإلكترونات بحرية من المعدن إلى الكاشف. قد يؤدي التصادم مع جزيئات الغاز إلى تقليل عدد الإلكترونات المكتشفة وتشويه النتائج.
يضمن الفراغ:
انتقال الإلكترونات دون عوائق
قياس دقيق للطاقة الحركية
بيانات موثوقة
ما الذي يجعل زجاج الكوارتز مناسبًا للتجارب طويلة المدى؟
يقاوم الكوارتز الهجوم الكيميائي وامتصاص الرطوبة. يبقى سطحه أملس ونقي حتى بعد سنوات من الاستخدام.
فوائد للباحثين:
انتقال مستقر للأشعة فوق البنفسجية
لا يوجد تدهور في السطح
نتائج متسقة مع مرور الوقت
كيف ينبغي للباحثين اختيار أنبوب الكوارتز المناسب؟
يطابق الباحثون درجة الكوارتز مع الطول الموجي المطلوب للأشعة فوق البنفسجية. يناسب الكوارتز من النوع الثالث تجارب الأشعة فوق البنفسجية العميقة، بينما يصلح النوع الأول للأشعة فوق البنفسجية القياسية.
نوع الكوارتز | الأفضل لـ | انتقال الأشعة فوق البنفسجية |
|---|---|---|
النوع الثالث | <220 نانومتر (الأشعة فوق البنفسجية العميقة) | >90% |
النوع الأول | 250-400 نانومتر | >90% |





